Authored by Gloria Yao*
Abstract
Development of electrochromic yarns opens up a high-tech option for the development of next-generation fashion textiles. Previous work has introduced electrochromic yarns comprising a metal wire core wrapped with organometallic compounds and conjugated polymers, but has reported unsatisfying colour-changing contrast and weavability. Here we report a tungsten trioxide (WO3)-electroplated high-contrast electrochromic filament suitable for weaving, and a 7-segment display embroidered with such filaments. The electroplating conditions have been optimised through elemental analysis via X-ray photoelectron spectroscopy (XPS), with the aid of coating morphology observation via scanning electron microscopy (SEM). We have learnt that sufficient WO3 for an evident change of colour can be deposited on the filament surface at -0.5V (vs. AgCl) in 30s. This low voltage and short coating duration have shown possibility for up scaled production of the electrochromic filament. Connecting the 7-segment display with ESP8266 Wi-Fi micro-controller unit, through remote signal input on ‘Blynk’ (an open-access mobile platform), we have demonstrated a double-digit ‘chance of rainfall’. Our study offers guidance on the design of textile-based information displays.
Keywords: Electrochromism; 7-segment display; Functional textiles; Tungsten trioxide (WO3)
Abbreviations: XPS: X-ray Photoelectron Spectroscopy; SEM: Scanning Electron Microscopy, ITO: Indium-doped Tin Oxide; TTIP: Titanium (IV) Iso-Propoxide; LD: Lethal Doses; SPA66: Shieldex® polyamide 6.6; SPA6: Shieldex® Polyamide 6; SSF: Stainless Steel Filament; PE-AL: Polyester 66wt%-aluminium 34wt%
Introduction
Optical modulation using electrochromic materials plays an important role in many areas, from the design of smart glass [1] to the development of adaptive camouflage films [2], and the next-generation textile fashion [3]. Classical applications of electrochromic materials include embedding metal oxides in glass for the reduction of solar heat and glare, and incorporating polymers or metal oxides in flexible substrates for the development of information displays [4]. In this study, we focus on the latter.
Electrochromic textiles are mainly produced by a. depositing conjugated polymers directly on indium-doped tin oxide (ITO) fabrics and b. fixing filaments comprising a core of conductive wire and a sheath of conjugated polymer in regular textile bases in situ. Although much effort has been put into enhancing contrast between colouration and bleaching of the textiles and achieving multi-coloured electrochromic features with negligible colouration degradation after hundreds of successive colouring-bleaching cycles, little attention has been paid to swift toggling between colouration and bleaching (timescale~5s) at low voltages and dermal toxicity of electrochromic polymers. Further, challenges such as the enclosing of fluidic electrolyte and the reduction of filament’s thickness and rigidity have not been overcome. All of these ‘factors’ have led to the scarcity of electrochromic yarns in commercially available textiles.
Production of electrochromic fabrics typically follows a ‘spray coating-chemical deposition’ method. In this method, ITO is spray-coated on textiles, making the textiles conductive. Polymers such as polyaniline, poly[2,3-bis(3,4-bis((2-octyldodecyl)oxy) phenyl)-5,8-bis(2,3-dihydrothieno[3,4-b][1,4]dioxin-5-yl)quinoxaline], 3,6-bis(dodecyloxy) thieno[3,2-b]-thiophene-dimethoxybenzene), occasionally doped with tungsten trioxide (WO3), are deposited on ITO-coated textiles to render the fabrics electrochromism [2,5]. The textiles show a range of colours in response to a series of voltage stimuli and exhibit no degradation of colouration after 150 colouration-bleaching cycles when bent and twisted [2]. The wide colouration range and the colouration stability under mechanical deformation make these textiles suitable for camouflage clothing. However, the 5-minute decolouration timescale hinders their application in real-time display.
Conventional electrochromic filaments have a ‘wire-polymer- electrolyte’ millimetric tri-layer structure. Stainless steel wires having a thickness in the order of 0.1mm are wrapped with polymers (i.e. poly (3,4-ethylenedioxythiophene), poly(3-methylthiophene) and poly(2,5-dimethoxyaniline)). Subsequently the ‘wire-polymer’ bilayers are coated with an electrolyte gel, thus forming electrochromic filaments (thickness ~0.3mm) capable of showing a series of colours for a range of voltages [4]. Although the submillimetric thickness of such filaments gives the filaments weaving and knitting potential, the 1-hour decolouration timescale limits the filament’s application in real-time displays. In addition, the filaments lack an enclosing layer for the electrolyte gel.
Research of soft electrochromic materials other than textiles can be found in literature. Using a conductive film and polymers synthesised via Suzuki-Miyaura cross coupling reaction between an aniline and several aryl dibromide compounds, Beaupre et al have invented a flexible electrochromic cell and visualised a series of colours as the voltage varies from 0.6 to 1V in a timescale of 60 minutes [6]. Similarly, Ahmed et al have produced an electrochromic co-polymer film and achieved smooth toggling amongst multi-colours for -1.5V~+1.5V [7]. These electrochromic materials have potential for military-grade camouflage because of the smooth transition between colouration and bleaching, but cannot be used for clothing due to the toxicity of synthesised polymers and the poor material breathability.
Metal oxides, especially WO3, have been used as an electrochromic coating in films, not only because of their colouration stability under stretch, but more importantly, because of their improvable colouration efficiency through modification of surface morphology. Previous work has modified WO3’s morphology by varying the concentration of urea and water-to-acetonitrile volume ratio in the WO3 coating solutions [8, 9]. Specifically, Urea governs the cross-sectional width, height and the growth density of the nanorod, while water-to-acetonitrile ratio regulates the circularity of nanorod. Research has shown that vertically oriented hexagonal- cross sectioned nanorods maximise the colouration efficiency because of the nanorod’s large tunnels and active surface area available for electrochromic reactions.
Swift colouration can be seen when the thickness of WO3 coating falls in a specific submicron range. For thermally annealed WO3 layer having thickness in the order of 0.6~1μm, colouration at -0.25V occurs in 20s. For WO3 layers either thinner than 0.6μm or thicker than 1μm, colouration does not contrast evidently with bleaching on a 20s timescale [10].
Metal oxides layers having an interconnected Nano porous structure and a marked ‘low pore aspect ratio’ can efficiently resist colouration degradation after thousands of successive colouration- bleaching cycles. WO3 layers with such characteristics can facilitate efficient proton migration and charge transfer, thus preserving the colouration performance over 2000 successive colouration- bleaching cycles [10]. The ‘low pore aspect ratio’ is a result of the scale separation between the pore size and the thickness of a metal oxide layer. For V2O5 layers, the 10nm pore size and the 20μm layer thickness result in an aspect ratio of 5∙10-4, thus preserving good ion intercalation stability after 250 colouration-bleaching cycles [11].
Traditional methods of depositing electrochromic metal oxide on rigid substrates require either high voltages or high temperatures. For example, V2O5 Nano crystals capable of showing shades of blue and yellow have been deposited on polished vanadium foils through room-temperature electrochemical anodisation at 120V [11]. Nano porous TiO2 coating (thickness~1μm), capable of showing shades of deep blue, has been deposited on fluorine doped tin oxide glass through a high-temperature assisted 12-hour hydrothermal treatment [12]. Although these methods produce coatings with attractive colouration performance, they lead to great cost in scaled production because of the high energy input and long treatment duration.
Deposition methods working at less extreme temperature and voltage have been developed. A method comprising spin coating of metal-precursor-based solutions on a fluorine tin oxide glass, followed by a UV-curing and oven annealing process, has been used for the preparation of multi-layered electrochromic films of WO3, MoO3 and V2O5 [13]. More recently, e-bean evaporation has been used for the deposition of grains of needle-shaped NiO nanocrystals, thickness in the order of 500nm, on films [14]. Although these methods produce electrochromic devices showing fast colouration (timescale: 6~10s), data of electrochromic layer deposition using these methods are only available for rigid substrates.
Deposition methods have been combined to produce metal oxide Nano arrays with unique configuration for high colouration efficiency. Combining self-assembly of polystyrene and electro deposition, with the aid of annealing at 200°C, Xia, et al. [15] prepared bowl-like Co3O4 Nano arrays with improved ion intercalation efficiency for colouration. However, forming such complex periodic configurations requires precise manipulation of self-assembly on surfaces with low roughness. The roughness preference limits the substrates to glass and silicon wafers.
Challenges of depositing metal oxide on textile yarns using the abovementioned methods include loss of yarn’s mechanical strength and decomposition of yarn at high temperatures. Yarns of cotton and poly (ethylene terephthalate) are particularly unsuitable for the abovementioned methods because they decompose at 100°C and 150°C [16,17]. Further, nylon 66 undergoes a yellowing process over 200°C that permanently damages the yarn’s visual attractiveness.
In this paper, to overcome these challenges, we employ a room temperature, low voltage electrodeposition method that enables energy-saving production of electrochromic coatings on yarns. In contrast to all the previous work on electrochromic textiles, we embroider the electrochromic yarns to a 7-segment display and demonstrate a mobile-app controlled double-digit numeral display.
Materials and Methods
In this study, the preparation of electroplating solution follows two popular approaches as disclosed in literature [18-22]: a. dissolving high-purity metal powders in strong oxidants to produce metal oxide solutions, followed by the addition of dilutants; b. directly dissolving powders of metal oxides or salts in solvents. Tungsten (W), titanium (IV) iso-propoxide (TTIP), sodium molybdate (Na2MoO4), nickel (II) chloride (NiCl2∙6H2O) and cobalt (II) sulphate (CoSO4∙7H2O) have been purchased from Sigma Aldrich and used without pre-treatment. The preparation procedures are briefly summarised below:
• WO3 solution: 1.8g W is mixed with 60ml 30 vol% H2O2 under vigorous stirring until a milky mixture is formed. A trace of Pt black (Sigma Aldrich) is added into the mixture to decompose the excess H2O2. Impurities are removed using filter papers and the solution is diluted with 2-propanol.
• TiO2 solution is prepared by dissolving 30ml TTIP in 60ml glacial acetic acid with stirring for 30 min at room temperature. 30ml deionised water is added to the solution and the stirring is resumed until precipitate disappeared.
• MoO3 solution is prepared by dissolving 5 mM of Na2MoO4 in distilled water.
• NiO solution is an aqueous nickel chloride solution containing 0.5 M nickel chloride (97%), 0.1 M KCl and a trace of EDTA (99%) as complexing agent.
• Co3O4 solution is prepared by mixing 100ml of 1 M cobalt sulphate, 60ml of 0.25 M potassium persulfate, 20ml of aqueous ammonia and 20ml of deionised water at room temperature.
Toxicity of metal is assessed by comparing the oral, inhalation and dermal lethal doses (LD50) of a powder to ratings on the Hodge-Sterner scale [23]. This scale classifies material toxicity into 6 ratings based on the measured LD50 to rats/rabbits, with 1 being extremely toxic and 6 being relatively harmless. The metal powders for this study have an oral LD50 of 5000 – 10000mg/kg, matching rating 5 on the Hodge-Sterner scale. Therefore, the powders are considered ‘harmless’.
To avoid getting rigid filaments like the ones in Ref [4], we have limited the yarn count to 50 tex. filaments of Shieldex® polyamide 6.6 (SPA66), Shieldex® Polyamide 6 (SPA6), stainless steel filament (SSF) and polyester 66wt%-aluminium 34wt% (PE-AL) are cleaned with distilled water and dried naturally at room temperature before experiments. Filaments of SPA6 and SPA66 are coated with a layer of 99% purity silver for electroplating.
Electroplating of metal oxides on the filament surface takes place in a three-electrode cell controlled by a Potentiostat system. To identify the voltage, temperature and duration that offer the thickest metal oxide layer, we have run the electroplating at -1.8 V~ -0.5 V (vs. Ag/AgCl) for 30-600s at temperatures of 22-70°C. The final current is limited to -21mA ± 0.5mA to avoid unnecessarily long electroplating time. After electroplating, the filaments have been rinsed with deionised water to remove residual electroplating solution and dried in a convection oven at 70°C for 3 hours.
X-ray photoelectron spectroscopy (XPS) is employed to study the atomic percent (at%) of metal electroplated on the filament surface. An XPS spectrum shows the number of photon electrons ‘knocked off’ by an electron beam as a function of binding energy (eV). In this spectrum, the peak height stands for the number of atoms of one element relative to other elements. Assuming that the material to be homogeneous and isotropic, the atomic fraction for element i in the compound is calculated via (Equation 1):
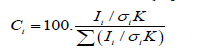
Where Ci is the fraction of element in %, I is the peak area of element i, σ is the photoionization cross-section of peak i, K is an instrumental factor.
Results and Discussion
Producing WO3-based electrochromic yarns via electroplating
The filaments used in this study are characterised by a multiscale structure and a gold or silver colour with metallic shine, as shown in figure 1A to 1D. All the four filaments have a diameter in the order of 200μm. Filament A to C are composed of small diameter fibres (diameter~30μm) twisted around the filament’s central axis, whereas filament D has a fibrous core wrapped by a polymer continuum (figure 1H).
According to literature [10], the white substance on the fibre surface, as shown in figure 1E-H, should be interpreted as amorphous WO3 coating. Visually SPA66 fibre (figure 1E) has been covered with the thickest WO3 layer. Examination of multiple spots on coated SPA66 show that the WO3 distribution illustrated in figure 1E can be seen frequently. The coating is particularly evident when an uncoated SPA66 (figure 1I) is juxtaposed with coated SPA66 (figure 1E). WO3 has formed only discrete particles on the surfaces of SPA6 (figure1F), SSF (figure 1G) and Al-PE (figure 1H), because SPA6, SSF and AL-PE have relatively high resistance per meter (>300Ω/m) (Figure 1).
SEM imaging of surface morphology for coated SPA66 filaments offers an idea of the thickness and the distribution of coating on fibres. Figure 2A-E show SPA66 fibre surfaces after electroplating in a series of metal oxide solutions at -0.8 V vs. Ag/AgCl for 150s. Co3O4 solution has formed the thickest layer on the fibre surface. Atomic percentage (at%) of an element in the top 20 atomic layers of a fibre implies the density of the element for an examined area, hence is used as an indicator of coating efficiency for a fixed electroplating duration in our study. Using equation 1, we found that Co3O4 has the highest coating efficiency (at%≈12%), followed by WO3 with at%≈8%. Other solutions resulted in low at%≤5% at this electroplating potential. Since the coating efficiency of Co3O4 and WO3 do not differ largely, in our discussion we only focus on WO3 (Figure 2).
2-propanol has been reported as a stabiliser for metal oxide- based electroplating solutions [24] but its concentration in solution must be carefully optimised. As the propanol’s volume percentage (vol.%) increases from 25% to 75%, the thickness of WO3 electroplated on SPA66 fibres decrease monotonically (figure 2FH). The addition of excessive 2-propanol raises the pH of the electroplating solution, leading to a poor electroplating efficiency for WO3. In our study 25vol% of 2-propanol has been used to maximise the electroplating solution stability without significantly compromising the coating thickness.
Atomic percent (at%) of W shows the amount of W normalised by the total amount of C, O, N and W for a local spot having a cross sectional diameter~200nm and depth~10nm from the surface, and the averaged at% over multiple ‘spots’ indicates the amount of WO3 coated on the SPA66 filament, assumed that WO3 is relatively evenly coated on all the fibres of an SPA66 filament. Using equation 1, we have obtained the at% of W for all the coating conditions in our study (Figure 3). The most amount of WO3 is obtained for -0.5V at 40°C and 600s coating duration.
Development of a 7-segment numeral display
Conventional electrochromic filaments are composed of a working electrode made of a stainless steel wire, layers of π-conjugated polymers, a poly (methyl methacrylate) (PMMA)-based Lithium electrolyte, and a counter electrode of a stainless steel wire twisted around working electrode’s central axis [4]. Such a design results in the filament diameter in the order of 2mm, making weaving and knitting difficult. Integration of such filaments in fabrics is limited to either fixing long individual filaments to a fabric base or manually aligning filaments in two orientations at 90° to one another [4]. The produced electrochromic fabrics show unnoticeable colour- changing performance. Further, the polymers are reported to be skin-irritating and consequently, have limited uses in wearable devices.
In our study, a 7-segment display is prepared by embroidering WO3-coated filaments (gold) in a cotton fabric (white), as shown in figure 4A1. The segment dimensions are L≅8.5mm and δ≅1.5mm. To simplify the denotation of surfaces, we refer to the surfaces in A1 and A2 as ‘front’ and ‘back’. At the back, each segment has a filament extending out-of-plane to connect the segment to the anode of a power source. A ‘bi-layer’ is prepared by fixing a dielectric membrane having the dimensions of the segment to a Cu strip with adhesive. The bi-layers are soaked in a 5wt% H2SO4/PVA gel (figure 4B1) before being gently placed on each segment using tweezers (figure 4B2). Thus, an assembly comprising a segment, a dielectric membrane and a Cu strip is formed. A thin Ag wire is carefully welded on the surface of the Cu strip to connect the strip to the cathode of the power source. Next, this tri-layer assembly is sealed with a transparent membrane at the front and back surfaces. The sealing slows down the dehydration kinetics of the electrolyte and packs the assembly tightly for maximum stability against repetitive manual handling during experiments. Segment’s colour changes from light gold to deep violet for a testing voltage of -0.8 V vs Ag/AgCl (figure 4C3). Figure 4C2 is juxtaposed with 4C3 for a clear contrast between colouration and bleaching (Figure 4).
To read more about this article........Open access Journal of Textile Science & Fashion Technology
Please follow the URL to access more information about this article
To know more about our Journals...Iris Publishers





No comments:
Post a Comment