Authored by Aamir Jalal Al Mosawi*,
Abstract
Background: There is no satisfactory or curative therapy for many of the disabling genetic disorders such as Ekman-Lobstein syndrome (osteogenesis imperfecta). However, it is hoped that advances arising from the accumulating research evidence can contribute to improving treatment of such conditions. Therefore, reviewing the literature for the recent research evidence is recommended to improve the therapeutic services for such disorders.
Materials and methods: A case of Ekman-Lobstein syndrome associated with significant disability because of the lack of effective treatment was studied. The medical literature was reviewed for the recent research evidence that may help in improving the therapeutic services for patients with Ekman-Lobstein syndrome.
Result: The occurrence of Ekman-Lobstein syndrome has been observed and reported in Iraq, but it has not been well documented. An Iraq boy aged three year with the main clinical manifestations of non-lethal form of this disorder is described. The boy had a significant disability because of the lack of satisfactory treatment. The boy had experienced multiple fractures since early infancy that made him crippled and unable to walk.
Conclusion: Literature review helped in recommending an evidence-based treatment which is intravenous bisphosphonates every few months, this treatment may contribute to some improvement of the management of Ekman-Lobstein syndrome.
Keywords: Ekman-Lobstein syndrome; Clinical and radiologic features; Therapeutic challenge
Introduction
Ekman-Lobstein syndrome; Clinical and radiologic features; Therapeutic challenge
• Lobstein syndrome. Vrolik syndrome.
• Fragilitas ossium Glass-bone disease.
• Brittle bone disease.
• Osteogenesis imperfecta.
Ekman-Lobstein syndrome or osteogenesis imperfecta is considered a group of genetic disorders of variable severity that affect mainly bones. The most important characteristic feature of osteogenesis imperfecta is fragile bones that are easily broken. The other important feature of the disorder is a blue discoloration of the sclerae of the eyes.
The disorder is associated with defective connective tissue formation and deficiency of type-I collagen resulting from an amino acid substitution of glycine to bulkier amino acids in the collagen triple helix structure. The larger amino acid sidechains cause steric hindrance resulting in a bulge in the collagen complex which disturbs the molecular nanomechanics and the interaction between molecules. The defective collagen is supposed to be eliminated by hydrolysis. However, failure to eliminate defective collagen results in abnormal relationship between the collagen fibrils and hydroxyapatite crystals leading to bone brittleness [1-5].
Materials and Methods
A case of Ekman-Lobstein syndrome associated with significant disability because of the lack of effective treatment was studied. The medical literature was reviewed for the recent research evidence that may help in improving the therapeutic services for patients with Ekman-Lobstein syndrome.
Result
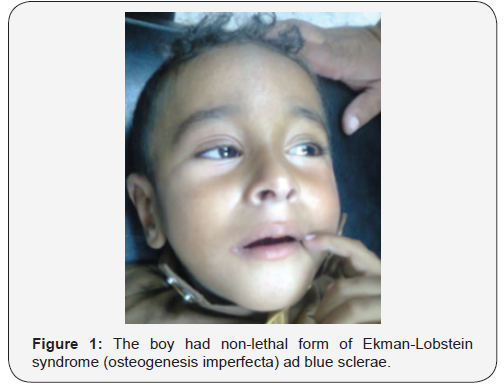
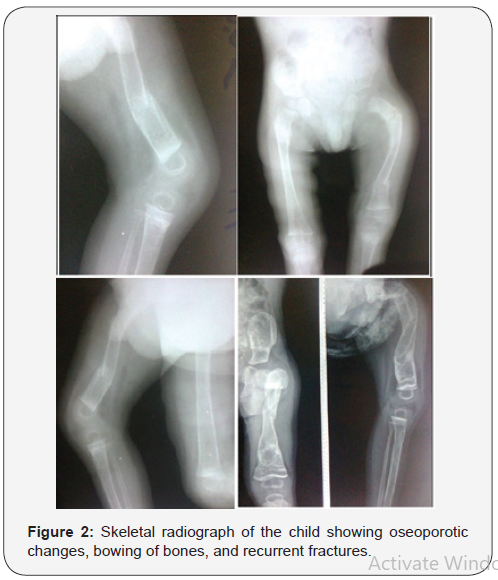
The occurrence of Ekman-Lobstein syndrome has been observed and reported in Iraq, but it has not been well documented [1,6,7]. An Iraq boy aged three year with the main clinical manifestations of non-lethal form of this disorder including blue sclerae (Figure-1), bowing of bones, and recurrent fractures was observed. The boy had a significant disability because of the lack of satisfactory treatment.The boy had experienced multiple fractures since early infancy that made him crippled and unable to walk. Figure-2 shows skeletal radiograph of the child which showed oseoporotic changes, bowing of bones, and recurrent fractures. Serum calcium of the child was 2.7 mmol/L (Normal: 2.1-2.6 mmol/L).
Eekman-Lobstein syndrome treatment research progress
There is no curative therapy for Ekman-Lobstein syndrome (osteogenesis imperfecta), and management strategies are generally aiming at increasing bone strength in order to prevent fracture and maintain mobility. During the late 1940s, the surgical insertion of metal rods in the long bones had been tried by Harold A. Sofield to improve strength at Shriners Hospitals for Children in Chicago. In 1959, Harold A. Sofield and Edward A. Miller published an influential article describing a useful treatment aiming at stabilizing and strengthening bones, and thus preventing fractures and improving rehabilitation [1]. There have been several clinical trials conducted with biphosphonates including pamidronate, alendronate, and zoledronic acid with the aim of increasing bone mass and reducing the incidence of fracture [8-14] (Figure 1,2).
Bisphosphonate treatment has been shown to reduce long-bone fracture rates, but such fractures remain frequent despite treatment. Although oral biphosphonates are more convenient and cheaper, they are not absorbed well, and intravenous bisphosphonates are generally more effective. Intravenous bisphosphonates has become the most widely used medical treatment for Ekman-Lobstein syndrome (osteogenesis imperfecta). Intravenous biphosphonate infusions has an important effect on vertebra in growing children and can help in vertebral reshaping after compression fractures. However, biphosphonates are less effective for preventing longbone fractures [1,2,3,11].
Pamidronate (Aredia) has been used in USA, UK and Canada. Pamidronate is usually given as an intravenous infusion, lasting for about three hours, and is generally repeated every three to six months and usually needed for the life. A clinical trial conducted by Glorieux et al (1998) suggested the effectiveness of intravenous pamidronate in severe cases osteogenesis imperfecta pamidronate as its use reduced bone pain, prevented new vertebral fractures, reshaped previously fractured vertebral bodies, and reduced the number of long-bone fractures. Common side effects of pamidronate include bone pain, low calcium levels, nausea, and dizziness [1,8.9,14].
Oral risedronate increased bone mineral densities and reduced non-vertebral fractures. However, it did not decrease new vertebral fractures [1]. A study conducted by Lv et al in 2018 showed that a once-yearly 5 mg infusion of zoledronic acid and weekly oral alendronate were associated with similar effects in increasing bone mineral density and decreasing bone resorption in children and adolescents with Ekman-Lobstein syndrome (osteogenesis imperfecta). However, zoledronic infusion was superior to alendronate in decreasing the clinical fracture rate [13].
Discussion
In 1788, the Swedish doctor Olof Jakob Ekman described the condition in his doctoral thesis and mentioned cases with condition of observed as early as 1678. In 1833, Jean Lobstein studied the condition and differentiated it from osteomalacia. He called the disease “idiopathic osteopsathyrosis.” In 1849, Willem Vrolik described the condition in a newborn infant with poorly ossified calvarium and called it osteogenesis imperfecta [1] The earliest classification of Ekman-Lobstein syndrome (osteogenesis imperfecta) included two main types [1,15,16,17,18,19,20]:
• Osteogensis imperfecta tarda which is the less severe type, and fractures are not present at birth.
• Osteogensis imperfecta congenita, a more severe form, and is associated with fractures at birth.
• However, several subtypes have been proposed during the previous decades.
• Bluish discoloration which is the result of thin sclerae showing the choroidal veins occurs in types I, type III,
• Severe lethal types include type II (usually lethal in the perinatal period, and first year of life); type VII, type VIII (associated with the protein lepreca)
• Autosomal dominant types include type I (60% de novo), type II (approximately 100% de novo), type III (100% de novo), type IV (60% de novo).
• Autosomal recessive includes Type VII and type VIII which caused by mutation in the gene LEPRE1.
Type I is generally mild as the collagen is of normal quality but no produced in sufficient amounts. Bones are fragile and broken easily. There may be slight spinal curvature, loose joints, hypotonia, and early loss of hearing may occur sometimes. Life expectancy can be slightly lower than general population because of the possibility of fatal bone fractures.
Type I without dentinogenesis imperfecta is considered type IA, while type IB is associated with dentinogenesis imperfecta which is characterized by opalescent teeth).
Type II is associated with severe deformity, insufficient quality and quantity of collagen, and most patients die within the first year of life due to respiratory failure because of underdeveloped lungs or intra-cerebral hemorrhage.
Type II has three radiographic patterns of the long bones and ribs including:
Type IIA is associated with broad and short long bones with broad and beaded ribs.
Type IIB is associated with broad and short long bones with thin ribs that have little or no beading.
Type IIC is associated with thin and longer long bones with thin and beaded ribs.
Type III is progressively deforming type as the symptoms are generally mild during the neonatal periods. It is associated with adequate amount of collagen but is defective. The bones are easily fractured, and fractures may occur before birth. Severe bone deformity often ensues, and respiratory problems may be encountered. The patients may have short stature, spinal curvature. Barrel-shaped rib cage, triangular face, loose joints, hypotonia in arms and legs, and early loss of hearing is possible. Lifespan may be normal, but severe physical handicapping is expected.
Type IV is associated with normal sclerae. The collagen is adequate in quantity but of a poor quality. Bones are fractured easily particularly before puberty. Bone deformity is mild to moderate. The patients may have short stature, spinal curvature, barrel-shaped rib cage, and may experience early loss of hearing.
Like Type I, Type IV without dentinogenesis imperfecta are considered type IVA, while type IVB is associated with dentinogenesis imperfecta.
Type V is clinically similar to type IV and its inheritance is unknown. However, histologically type V is associated with “meshlike” bone appearance.Type V is also characterized by the “V Triad” which includes: 1-Radio-opaque band near the growth plates. Hypertrophic calluses at fracture sites because of abnormally high amounts of repair tissue. Calcification of the radio-ulnar interosseous membrane causing difficulty in turning the wrist.
Type V is also characterized by the “V Triad” which includes: 1-Radio-opaque band near the growth plates. Hypertrophic calluses at fracture sites because of abnormally high amounts of repair tissue. Calcification of the radio-ulnar interosseous membrane causing difficulty in turning the wrist.
Type VI has unknown inheritance and similar clinical features to type IV, but has unique the histological findings called fish scale bone appearance [1,15,16,17,18,19,20].
Conclusion
Literature review helped in recommending an evidencebased treatment which is intravenous bisphosphonates every few months, this treatment may contribute to some improvement of the management of Ekman-Lobstein syndrome.
To read more about this article...Open access Journal of Orthopedics Research
Please follow the URL to access more information about this article
To know more about our Journals...Iris Publishers
To know about Open Access Publishers





No comments:
Post a Comment