Authored by Mindong Chen*,
Abstract
In recent decades, cyclic peptides have attracted great attention due to their unique properties. In this paper, novel cyclic hexapeptide (6) and octapeptide (9) were designed and synthesized using liquid phase synthesis methodology. They were characterized by FT-IR, 1HNMR spectroscopy, LC-MS, scanning electron microscopy (SEM) and atomic-force microscopy (AFM). We have used these cyclic peptides as novel building blocks to generate porous materials by self-assembly.
Keywords: Cyclic hexapeptide; Cyclic octapeptide; Self-assembly
Mini Review
Chemists have isolated and identified a large array of cyclic peptide compounds from nature, particularly from plants and marine organisms, since the first one having biological activity, the cyclic peptide antibiotic gramicidin S, was discovered in 1974 [1]. Recently, there has been a number of excellent works disclosed regarding synthetic cyclic peptides [2,3]. Cyclic peptides may be classified as homodetic cyclopeptides, wherein the ring is composed of standard peptide bonds, and heterodetic cyclopeptides in which one or several links are not peptidic. A characteristic property of cyclic peptides is their stability conferred by the absence of N- and C-termini and a constrained backbone [4,5]. Based on this, cyclic peptides play an increasingly important role in bio simulation [6], drug design [7-9], nanophase materials [10], biosensors [11], catalysis, material science [12-23] and bone tissue engineering [24,25].
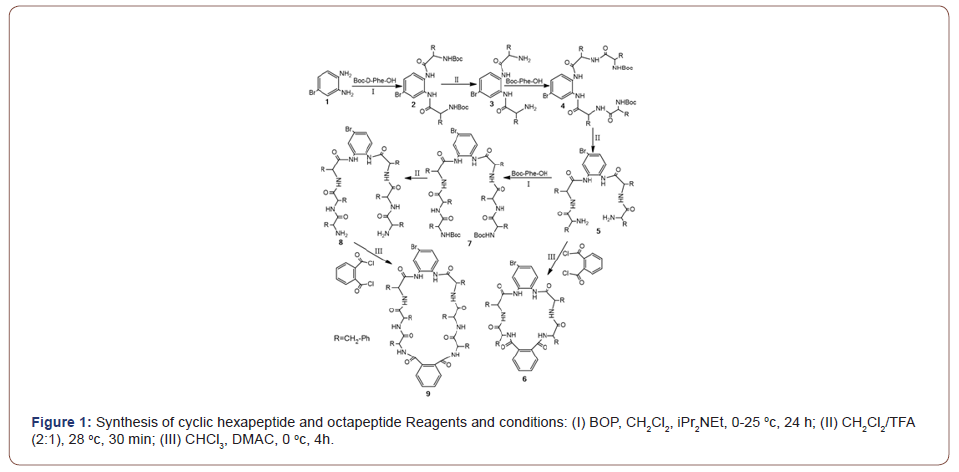
In this paper, we present some studies in the rapidly developing field of synthesis of cyclic peptides, utilizing self-assembly with conventional as well as some more recently developed initiators. Herein, we report the synthesis of two novel phenylalaninebased cyclic peptides and their self-assembly properties. All reactions were carried out in liquid phase and proceeded with moderate to good yields (Figure 1) [18]. Dimethylacetamide (DMAC) was used as solvent and also as the catalyst for cyclization. We used benzotriazole-1-yloxytris (dimethylamine) phosphonium hexafluorophosphate (BOP) as the condensing agent and trifluoroacetic acid (TFA) as the deprotecting reagent.
Compound 2 was prepared by condensation of 4-bromo- 1,2-benzenediamine and Boc-D-Phe-OH under nitrogen in good yield (68%) and, following deprotection with 30% TFA in DCM gave compound 3 in a yield of 78% after simple recrystallization. Compound 3 was then repeatedly condensed and deprotected by similar procedures to afford 5 and 8 in acceptable overall yields (92%, 29%). The desired cyclic hexapeptide 6 was synthesized by treating the corresponding precursor 5 with 1,2-benzenedicarbonyl chloride under mild conditions in a moderate yield (29%), and the same procedure gave cyclic octapeptide 9 in a yield of 21% [26]. The structures of compounds 2-9 were characterized by FT-IR, 1H NMR spectroscopy and LC- MS. The final product 6 showed molecular ions at 904, 906 and 9 at 1198 and 1200, corresponding to [M+Na]+Br79-Br81 respectively. Resonances in the 1H NMR spectrum could be fully assigned and the 1H NMR and FT-IR spectra of 2-8 were also in agreement with the proposed structures. Partial assignments are given in the experimental section [26].
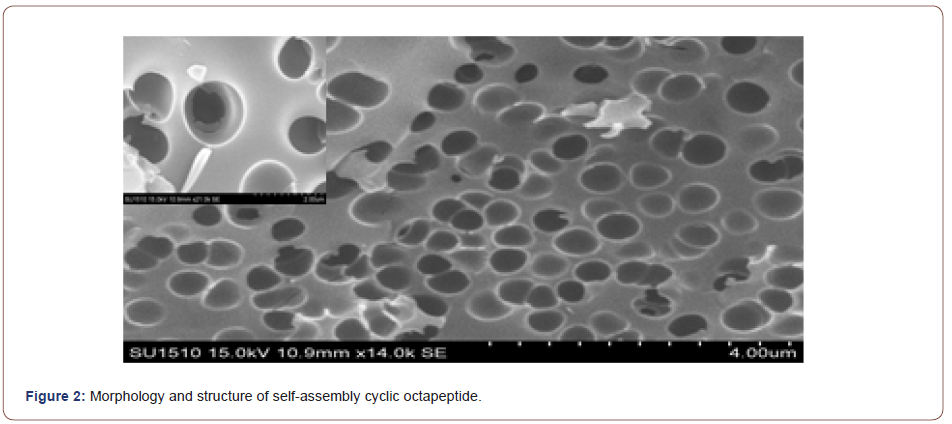
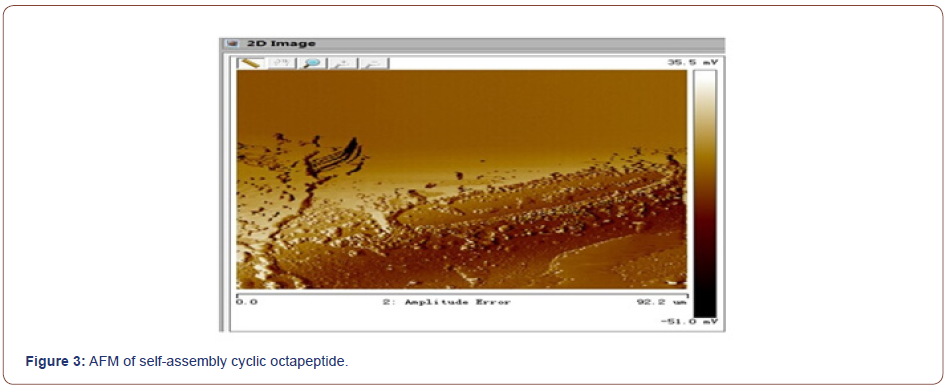
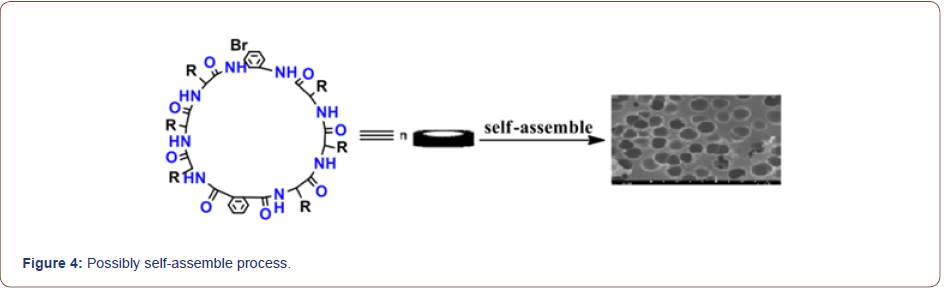
Self-assembly studies were performed by dissolving 10 mg of the cyclic peptide in 1 mL neat trifluoroacetic acid (TFA) and placing the resulting solution in an open 2 mL Eppendorf tube. The Eppendorf tube was then floated in 30 mL of double-distilled (dd) H2O in a 45-mL conical tube. The two samples were allowed to equilibrate for 48-72 h, after which the Append or f tube was removed and found to contain a heavy suspension of microcrystals. The crystals were recovered quantitatively by centrifugation and washing with ddH2O or by dialyzing against ddH2O to remove any remaining TFA. FT-IR spectroscopy indicated that TFA did not participate in the crystal lattice and the morphology and structure of the self-assembled cyclic peptide 9 were characterized by scanning electron microscopy (Figure 2, 3). The SEM images indicated that the self-assembled material derived from cyclic octapeptide 9 was porous and sheet-like. The AFM image showed that average diameter of the holes was 0.90μm, with an average depth of about 1.5~2.0μm. Possibly self-assemble process was shown in Figure 4.
In conclusion, we have reported the liquid phase synthesis of a cyclic hexapeptide and a cyclic octopeptide based on phenylalanine linked with o-phenylenediamine and phthalic acid caps. The advantage of this approach is that the reaction can be performed by simply mixing starting materials, acid and capture agent, giving moderate to good yields under mild conditions. A self-assembled porous material was also obtained in a good yield under mild conditions.
To read more about this article...Open access Journal of Modern Concepts in Material Science
Please follow the URL to access more information about this article
To know more about our Journals...Iris Publishers
To know about Open Access Publishers





No comments:
Post a Comment