Authored by Elizabeth H Silvy*,
Abstract
Aquaculture is vital for supporting the United States (U.S.) seafood production and to meet the growing demand for seafood and lessen reliance on foreign imports. The U.S. is turning to novel technologies and refining methodologies in aquaculture production to meet these demands. Especially, there is a clear lack of warm-water, marine-culture facilities in the U.S. To address the needs of the growing aquaculture industry, we conducted research to evaluate the use of a slow-release, cellulose- based spawning peptide implants containing sGnRHa combined with an intramuscular sGnRHa peptide and domperidone injection would synchronize and induce ovulation in captive, sexually mature female cobia. Five adult female cobia were received a combination of sGnRHA Ovaplant® implants and Ovaprim® injections that were successful in inducing ovulation under natural spawning season photoperiod and temperature without intensive photo-thermal manipulation. The induction of ovulation through hormone injection in sexually mature adult cobia was successful. The lack of fertilized eggs produced in this trial indicated that environmental conditioning as well as hormone injections may prove to be more successful.
Keywords: Aquaculture; Cellulose-based; Cobia; Induced ovulation; Rachycentron canadum
Introduction
Cobia (Rachycentron canadum), also referred to as ling and lemonfish, is a popular recreational sportfish, an internationally, commercially cultured food fish, and has tremendous potential as a domestically cultured species in the United States. Cobia is a high priority species for aquaculture in the Gulf of Mexico, the Southeastern U.S. coast, the Caribbean, and the subtropical/tropical waters of the Atlantic coast of South America [1]. Due to low supply, high market demand in price ($37.40/kg of fillet), fast growth rates, relatively low larval mortality compared to other marine species, and good feed conversion rates, cobia has been a fast growing sector for aquaculture in Asian and South and Central American countries such as Taiwan, South Korea, China, India, Panama, Brazil, Belize and the United States [2-4].
Cobia is a prime candidate for aquaculture for commercial foodfish production as well as stock enhancement programs to increase wild stocks for harvest. Little data exists on the population status of cobia, because they are primarily a recreational fish due to their solitary, pelagic life history that makes commercial harvest economically unfeasible, resulting in a limited commercial industry. In the limited commercial industry, it is easy to track the decline of cobia populations. In 2014, 18,143.7 kg of fresh and frozen fillets were harvested for the commercial market, down 3,638.7 kg from 2013 [5]. Recreational landings, either purposeful, or as by-catch, comprised 90% of total landings. In 2013, 54,431.0 metric tons of fish were recreationally harvested [6]. The System for Electronic Document Analysis and Retrieval [7] estimated total abundance and recruitment of cobia was below average following an increase in stock abundance in the early 2010s. Estimated stock abundance declines were due in part to recreational and commercial by-catch [7]. Stock enhancement programs for cobia in Mississippi, South Carolina, and Florida have shown varying degrees of success to bolster total abundance and recruitment [8]. Between 2001 and 2009, South Carolina’s Department of Natural Resources released 64,768 cobia fingerlings [8]. These numbers are significantly lower than that of red drum (Sciaenops ocellatus) stock enhancement programs. More than 33 million red drum were released in 2010 [8] and over 735 million have been released in Texas as of August 2018 (Robert Vega, Texas A&M University–Corpus Christi, Texas; personal communication, 2019).
The earliest known attempt at spawning cobia in captivity occurred during the 1970s in North Carolina, in which researchers harvested gametes from wild caught broodstock and failed to produce juveniles from the larval stage [9]. Further research efforts have been made to spawn cobia in captivity in Florida, Mississippi, South Carolina, Texas, and Virginia [10]. Many of these efforts attempted to induce spawning using injectable human chorionic gonadotrophin (hCG) or slow-release implants (salmon gonadotropin releasing hormone analogues, sGnRHa; [11]). Several attempts to spawn cobia were successful using hCG or luteinizing hormones but failed to optimize larval production and resulted in poor survival [9,10].
In the early 1990s, researchers at the University of Texas began rearing wild caught juvenile cobia in captivity to maturity. Researchers documented the first volitional spawning of cobia in 2002 using water temperature and photo-period manipulation [12]. However, only a single pair of a larger group of cobia was documented to spawn. Volitional spawning brought on by only environmental conditioning has proven to be sporadic and unreliable leading to eventual abandonment of some U.S.-based cobia research programs. Most successful spawning of cobia has been due in part to induced spawning of small numbers of broodstock through spawning peptide application, or by capture of mature spawning adults during the natural spawning season [11-13]. Due to the scarcity and complications associated with broodstock capture, many research projects have used small numbers of adult fish to accomplish spawning trials [11-13]. Successful spawning of adult cobia have been accomplished using the implantation of pelleted hCG (275 IU/kg BW; Franks et al. 2001), and with hormonal injection of 10 μg/kg luteinizing-releasing hormone analog (LHRHa, des-Gly10, and D-Ala6; Sigma-Aldrich, Singapore; Nguyen et al. 2010), but only in small numbers.
As university researchers and commercial hatcheries investigate the potential of novel species for culture, there is a need for research to investigate the ability of salmon gonadotropin-releasing hormone (sGnRha) implants and injections combined with environmental conditioning to induce spawning in captive cobia broodstock. Salmon gonadotropin-releasing hormone implants are commonly used to induce oogenesis and spermatogenesis in various marine and freshwater species such as Atlantic salmon (Salmo salar), Chinook salmon (Oncorhynchus tshawytscha), common snook (Centropomus undecimalis), Atlantic croaker (Micropterus undulates), Arapaima (Sudis gigas), common carp (Cyrprinius carpio carpio), and Atlantic cod (Gadus morhua) with high degrees of successful spawning [14-18]. The use of sGnRha peptide implants could alleviate bottlenecks preventing cobia production on a commercial scale by increasing cobia spawning success, eliminating seasonal availability, and establishing a steady supply. Improved spawning success also could alleviate capture pressure on wild stock through stock enhancement programs.
Objectives and Hypotheses
The primary objective of this study was to determine if slow-release, cellulose-based spawning peptide implants containing sGnRHa (Ovaplant®) combined with an intramuscular (IM) sGn- RHa peptide and domperidone (dopamine inhibitor) injection (Ovaprim® Syndel International Inc., Qualicum Beach, British Columbia, Canada) could synchronize and induce ovulation in captive, sexually-mature female cobia. The null research hypothesis was that sGnRha implants have no effect on synchronization and inducement of ovulation in female cobia.
Materials and Methods
Broodstock collection
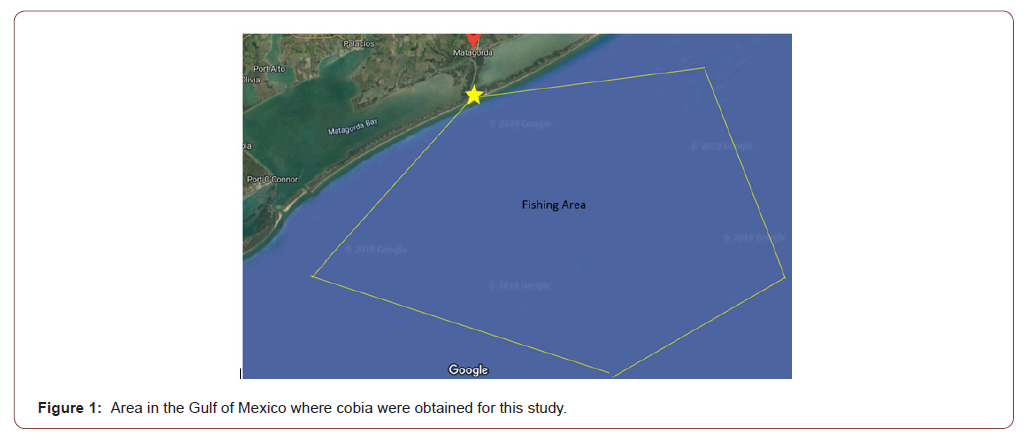
Sexually mature adult cobia (n = 5, mean length 0.873 m, mean weight 7.18 kg) were captured from the Western Gulf of Mexico cobia population using hook and line. Broodstock acquisition trips were conducted on a 7.62 m TransCat boat, captained by a local guide. Three collection trips were made, with cobia capture between 11.3 and 27.4 km offshore near oil platforms (Figure 1). The boat first circled the rig and then lines were dropped and jigs were used to entice cobia. Cobia were captured with traditional hook and line using jigs and live croaker purchased at a local bait shop. Other baits such as frozen ribbon fish and crab were used but did not result in cobia capture. Jigs were the initial bait that attracted cobia and when the first fish was hooked and brought to the surface, the schooling nature of the species brought companion fish to the surface. A live croaker on an unweighted line was then thrown in front of companion fish, resulting in multiple fish being captured at a single oil rig. During this process and due to the unreliable nature of hook and line fishing and user ineptitude, some fish broke off the line or escaped capture. Fish were then guided into a large net and brought aboard the boat and the hook was removed. Permits for collection of cobia broodstock from state waters were obtained from the Texas Parks and Wildlife Department (SPR- 0718-210). Hook and line were the most efficient and least stressful manner to capture large solitary cobia in the wild and resulted in the least amount of physical damage to the fish compared to other capture methods such as nets, trawls, and long lines. Cobia broodstock were immediately transferred into a double walled, insulat ed, 833-L shipping tote that contained 662-L of ambient seawater (approximately 32-24 g/L salinity) located on the flat front deck of the boat. Supplemental oxygen was provided to the shipping tote at 2-L/minute (Regulator; Roscoe Medical, RMI-15H CGA-540 H Regulator, 0-9 LPM Compass Health Brands, 6753 Engle Road Middleburg Heights, Ohio) from a 6.23-cubic meter compressed oxygen cylinder and fine pore, porcelain air stones (69.9 cm x 8.5 cm, Point Four™ Micro Bubble Diffusers, Pentair Aquatic Eco-Systems, Inc., Apopka, Florida). Multiple fish were collected before the boat returned to shore.
Broodstock transport
Cobia broodstock were transported to the Aquacultural Research and Teaching Facility (ARTF; 13950 FM 60 East, Somerville, Texas) in a 1,325-L-insulated-hauling tank. The insulated hauling tank was filled with 33 ppt seawater prior to onboard collection and temperature was uncontrolled but recorded at 25° C. Hauling tanks were provided oxygen from a 6.23-cubic meter compressed oxygen cylinder and dissolved oxygen (DO) was provided at 2-L/ minute. These values ensured acclimatization between boat tank and hauling tank. Vitalife® (Syndel International Inc., Qualicum Beach, British Columbia, Canada) slime coat was added to the tank water to prevent mucus build up and surface slime that could potentially limit oxygen consumption. These procedures were intended to limit stress on fish.
Broodstock acclimatization
Broodstock were then transferred from the haul tank to a black colored 1,000-L tank, with oxygen provided from a 6.23-cubic meter compressed oxygen cylinder at a rate of 2-L/minute, for a formalin dip to rid fish of parasites. Fish were transferred one at a time and weight and length were recorded. After 2 minutes in the formalin dip (25 mg/L), broodstock were moved to a 4,429-L recirculating tank system that was 27° C, 33 ppt and had a DO of 6.4 mg/L. Compressed oxygen at a rate of 2-L/minute was provided for 48 hours after transfer into the recirculating system using a compressed oxygen cylinder and fine pore, porcelain air stone (69.9 cm x 8.5 cm, Point Four™ Micro Bubble Diffusers). Fish were acclimatized to a photoperiod of 14 h of light and 10 h of dark with water temperature ranging from 26-30° C to simulate natural spawning conditions. System salinity was maintained at 28–32 ppt with artificial sea salt (Red Sea Salt, Red Sea North America, Houston, Texas). Dye (Aquashade®, Arch Chemicals, 501 Merritt 7 Norwalk, Connecticut) was added to the water of the tank to more easily acclimatize the fish to a lighter tank environment.
Five adult cobia were used in this experiment. Due to the relatively small size of the tank, the difficultly of replicating large recirculating systems, the projected egg production, with a single female having the ability to produce 10-20 million eggs during a spawning season (Kilduff et al. 2002), and comparable studies using similar or smaller numbers of brood stock [11-13] this number was considered adequate for the trial.
Broodstock care and feeding
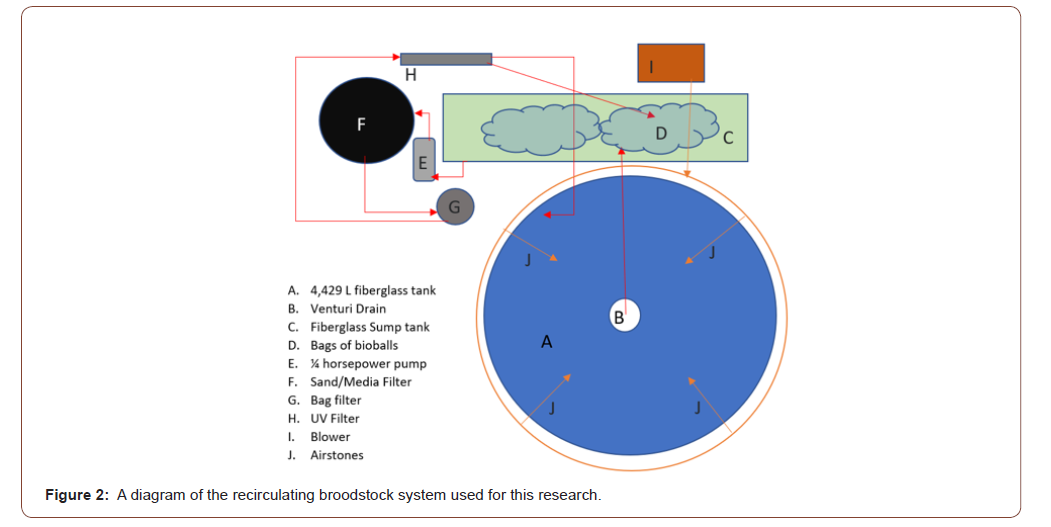
Broodstock were kept in a 4,429-L recirculating tank, at a photoperiod of 14 h of light and 10 h of dark photoperiod and at a water temperature of 26-30° C to simulate natural spawning conditions for the duration of the trial. The recirculating system consisted of one 4,429-L tank. The system includes a sump tank filled with two sacks of bioballs/filtration media for water collection, a 2-horsepower pump (Hayward® MaxFlo XL 2 HP Dual Speed Pool Pump, One Hayward Industrial Drive Clemmons, North Carolina), a Arias 4000© (Pentair Aquatic Eco-Systems Inc. 2395 Apopka Blvd. Apopka, Florida) bead filter, a 25-μm Water Co.© filter (Water Co.®, Augusta, Georgia), and a Jebao© PU-36 UV clarifier (Jebao®, Dongsheng, Zhongsha, Guangdong, China). One half of the water that exited the UV filter was returned to the sump tank. The other half of the water that exited the UV filter returned to the tank at an angle encouraging uni-directional waterflow in a circular pattern. The tanks were set up with a venturi drain style standpipe, covered in large mesh that let waste water flow into a drainage pipe that deposited waste water into the sump tank for filtration (Figure 2) Aeration was applied using four air stones from an external blower (Whitewater® Regenerative Blower Pentair Aquatic Eco-Systems Inc. 2395 Apopka Blvd. Apopka, Florida).
Broodfish were fed a mixture of frozen shrimp trawl bycatch, freshwater lake survey bycatch (Texas Parks and Wildlife Department, District 3 Fisheries Biologist Office, Sommerville, Texas), and live baitfish (Bait Barn Fisheries, Bryan, Texas) daily until satiation. Temperature, dissolved oxygen, salinity, and pH were monitored and recorded daily. Temperature was maintained at 26-30° C using heaters (Finnex®, HMA-S 100-Watt Titanium Heater, 360 Aquatics, 5710 Brittmoore Rd #9, Houston, Texas) located in the sump tank of the system. System salinity was maintained at 28–32 ppt with artificial sea salt (Red Sea Salt, Red Sea North America, Houston, Texas). Total ammonia nitrogen, un-ionized ammonia, and nitrite were determined and recorded bi-weekly. After 1 week in the recirculating tank system it was observed that fish were prone to jump out of the recirculating tank system, thus a large black mesh shade was then placed over and covered 90% the tank system.
Hormone implantation
After a 3-week acclimatization period to the system, fish were removed from the tank, sedated in an isoeguenol bath (Thermo Fisher Scientific Chemicals, Inc., Ward Hill, Massachusetts), and weight and length of each fish was recorded to calculate appropriate spawning peptide dosages. Each broodfish was administered 250 μg Ovaplant® hormone implants in the dorsal musculature anterior to the dorsal fin to achieve a final dosage of >50 μg/kg of body weight, and injected with Ovaprim® in the dorsal musculature on the opposite side of the implants anterior to the dorsal fin at a dosage of 25 μg/kg of body weight. These are standard dosages for spawning large fish determined from the product labels and were discussed with and confirmed by the product manufacturer (Dr. Peter McKenzie, Senior VP Product Management, and Dr. Katie Haman, DVM, Director for Spawn Products, Syndel, Ferndale, Washington). After injection, the fish were immediately returned to the tank and allowed to spawn volitionally within the tank.
Egg collection and ovulation assessment
An egg harvester made of 420-μm mesh was placed under the tank outflow pipe and monitored for eggs from 3 to 7 days, post-injection. Egg collection chambers were checked every 6 hours for presence of eggs. Date and time of egg collection was recorded when eggs were obtained from the collectors. When eggs were present, they were collected from egg collector using a 1,000-mL pitcher. The total quantity of eggs was extrapolated from 1 mL egg counts taken from 1L collections out of the egg collection chamber. The number of 1L collections also was recorded. Visual observations were made of egg size, quality, and clarity.
One thousand eggs per liter were incubated in each of 16, 113- L recirculating incubation tanks. The incubation system included a sump tank for water collection, a 2-horsepower pump (Hayward®), a sand filter (Arias 4000©), a canister filter (Water Co.©), and a UV filter (a Jebao©). One half of the water that went through the system exited the UV filter and was returned to the sump tank. The other half of the water that exited the UV filter returned to the tanks. The tanks were set up with an internal standpipe covered in 250-micron mesh that led to an external standpipe that maintained water level and let wastewater flow into drainage pipes that deposited wastewater into the sump tank for filtration. Temperature was controlled by a chiller/heater (Aqualogic®, 9558 Camino Ruiz, San Diego, California). Temperature was maintained at 26° C, salinity maintained between 28-32 ppt, and DO was kept above 4 mg/L. Incubation tanks were set for a 12-hour light and 12-hour dark cycle schedule. Aeration was maintained at a low level for the duration of the experiment. Animal Use Protocol 2016-0279 was followed for this experiment.
Data Analysis
Statistical analysis of this study was not possible due to the limitations of equipment, facilities, and numbers of broodfish necessary to have replicates for statistical analysis. This was extremely common for this type of study in which only small populations of large broodfish can be obtained and safely cultured by most research and commercial facilities. These types of studies are typically of more importance to biological and commercial significance than statistical significance. This was exemplified by other studies of this nature including published studies by the University of Texas in which a single spawning event by a single pair of cobia broodstock was publishable due to its significance to the culture and potential production of this species [10,11]. Therefore, this study represents an observational study in which biological and commercial significance, in the form of successful induction of ovulation and ability to obtain cobia eggs, was the criteria used to evaluate the success of the study. Obtaining eggs from a successful ovulation event was considered to be a positive test, while failure to induce ovulation or to obtain eggs was considered failure of the treatment.
Results
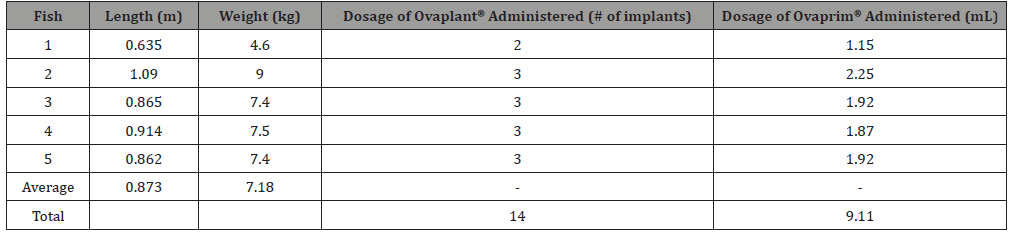
Five cobia were used in this study. This was comparable to other studies that have used the same number of fish or less [11,19]. Cobia captured for this study averaged 7.18 kg in weight and 0.873 m in length and hormone quantity was correlated to weight (Table 1). A total of 14 Ovaplant® hormone implants and 9.11 mL of Ovaprim® was used on the five fish. Immediately following hormone implantation, fish were lethargic and had to be manually held in oxygen flow for 1–5 minutes before resuming normal behavior. Two days after implants were administered lesions were visible at injection sites in two of the cobia.
Approximately 120 hours after injection at 0800 hours on July 30, 2018 eggs were observed in the egg collector. It was assumed that eggs were spawned overnight on the July 29, 2020. Eggs were collected for 2 hours from egg collection chamber and eggs were we observed floating in the broodstock tank. Cobia were observed to be circling the tank during egg collection. Water temperature was 28° C and salinity was 29 ppt at the time of egg collection. Eggs were small, approximately 1.5 mm in diameter, milky white in color and did not display any observable clarity. A large percentage (~80%) of the eggs were resting on the bottom of the collection chamber while the rest were floating on or near the surface of the water column (Figure 3).

Table 1: Length, Weight, and Hormone Injection Information for Individual Cobia Used in this Research.
There were approximately 330 cobia eggs per milliliter and 12,000 ml of the eggs were collected. Extrapolating on this enumeration indicated a total of 4 million eggs were produced (eggs from females combined). Eggs were divided equally and placed into 18 incubation tanks with light aeration and 100% daily water exchange. Mean batch fecundity was enumerated and estimated to be 800,000 eggs per female based on the assumption that all of the fish were female and ovulated.
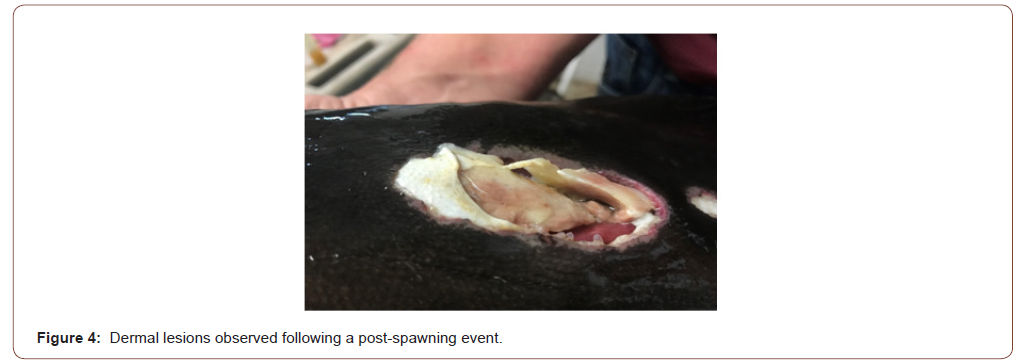
Twelve hours after estimated egg production, eggs were inspected under a microscope. Eggs were malformed and displayed signs of fungal infection. The specific fungal infection was not identified. All five fish survived the spawning event, but two were clearly sick or infected and displayed large dermal lesions located at injection sites (Figure 4). These fish were lethargic and rested on the bottom of the tank. These two fish were euthanized after the spawning event.
Discussion
The results of this study demonstrate the combination of sGn- RHA Ovaplant® implants and Ovaprim® injections were successful in inducing ovulation in female cobia under natural spawning season photoperiod and temperature without intensive photo-thermal manipulation. This was similar to previous studies that used photoperiod and/ or hormonal manipulation to induce ovulation [11,12,20]. Biesiot et al. [21] and Franks et al. [11] used human chorionic gonadotropin (HCG) injected at 275 IU/kg of body weight in female cobia to produce fertilized eggs after about 42 hours post-injection. Nguyen et al. [22] used injection of 20 μg/kg LHRHa and egg production was observed within 1-hour post-injection. This differs from our observations in that egg production in this study was observed 120 hours post-injection. This could potentially be due in part to lack of males, whereas males were identified and present in both of the aforementioned studies. The lag between injection and spawning also could be attributed to the time release nature of Ovaplant®.
In prior research by Arnold et al. [12], Kilduff et al. [20], and Benetti [23], cobia were spawned successfully using rigorous photothermal regimes. Water temperature manipulation as the only form of induce spawning manipulation has been proven to induce spawning in cobia [24] and other species such as red drum (Sciaenops ocellatus; [25]) and Nassau grouper (Epinephelus striatus; [26]). This was a viable manipulation to induce spawning but was not practical at a large commercial scale; whereas, hormonally induced spawning using sGnRHa, hCG, or LHRHa allows for out of season spawning without intensive temperature control.
Batch fecundity in this study was comparable to other smallscale hormone-induced-spawning studies. Franks et al. [11] observed 3.2 million eggs from two females 42 hours post-injection. While the results of this trial are slightly lower, if calculated by eggs per female, this could be attributed to the fact that Franks et al. [11] included a sexually mature male in their study that could have induced ovulation or there was a smaller lag time between injection and the ovulation event. Previous research conducted by spawning cobia without hormones indicate higher batch fecundity. Weirich et al. [19] used no hormone injection and stocked three male fish with two female fish and had a mean batch fecundity of two million eggs per female. This was higher than our observed batch fecundity and could be due in part to intense photothermal manipulation used to induce spawning.
In this research, eggs were not analyzed for a specific type of fungal infection. Fungal infections in pelagic finfish species eggs can be attributed to a multitude of fungi such as Achyla polyandra, Saprolegnia ferax, S. parasitica, S. diclina, S. australis, S. furcata, S. hypogyna, and S. unispora [27-29]. The fungal infection was thought to be caused by a lag in egg production and egg evaluation, improper water filtration, damage during egg collection in the collection chamber, or fungus present in the sump tank where the egg collection chamber was located. The common remedies to fungal infections include a disinfectant dip in 5% iodine immediately after egg collection, and/or hydrogen peroxide treatment [30,31]. Preventative care, adequate nutrition, prophylaxis and probiotics can all contribute to the success of healthy egg production.
Also noted were the presence of lesions caused by the repeated injections with the Ralgun® implanter that was necessary to achieve the desired dosage of Ovaplant®. Lesions were observed in two of the five fish injected at the injection site. A secondary bacterial infection led to the deterioration of the dermis and sub dermal musculature and eventually mortality. Negative side effects of Ovaplant® implantation have been observed in other species. Sink et al. [15] found that post-implantation of Ovaplant® in Atlantic croaker (Micropogonias undulates), injected female fish became lethargic and did not resume normal swimming behavior and mortality occurred in three of the 15 implanted fish. Hill et al. [32] found that negative effects of Ovaprim® injection included redness and bruising at the injection site and stemmed from degradation of water quality while holding and handling fish. Multiple studies have used Ovaplant® and Ovaprim® to induce gonadal maturation and spawning and have reported no adverse effects [14,33-36]. It was presumed the lesions and subsequent bacterial infections that led to lethargy were caused by repeated hormone injections needed to achieve the appropriate dose per body weight of Ovaplant®. Secondary bacterial infections could be associated with the nature of bacteria build up in a recirculating system or bacteria found naturally on the dermis of the fish. Further investigations need to investigate new methodology for the delivery of sGnRHa. A less invasive injection procedure would tamper many of the adverse effect caused by the use of a Ralgun® implanter as well as the need for multiple injections to meet the required dosage.
While this research was considered biologically and commercially successful from the predetermined standards evaluation, subsequent repetitions of the trial and further studies using sGnRHa implants and injections should investigate stage of sexual maturation and gender prior to injection. The inclusion of a known male in spawning trials may be pertinent or just a coincidental effect on successful spawning behavior. However, known males were identified and included in recorded successful spawning trials [1,11,37]. To assess stage sexual maturity and gender, ovarian samples should be aspirated from the fish prior to injection [11]. Weirich et al. [19] sedated fish prior to introduction in tanks and ponds and applied pressure to the abdomen of the fish to detect presence of running milt to identify males, while females were subjected to an ovarian biopsy to determine oocyte stage of development and size. These methods do have adverse effects such as exposure to harmful bacteria and increased handling time which was shown to increase stress and elevate cortisol levels which can delay or prevent successful spawning [15].
Alternatively, given the cobia in the Western Gulf of Mexico spawn every 7-12 days [38], it should be noted the specimens used in this research should all have been sexually mature, due to size, and in at least the early stages of previtellogenesis, due to collection time correlating with spawning season, and the injection of sGnRHa aided in successful spawning. While timing aided in the successful spawning event, to elucidate out of season spawns, sexual maturity and gender must be assessed before injection. There should be further investigation into out-of-season spawning based exclusively on hormone injection.
As with any cultured species, the success of spawning was dependent on the health and sexual maturity of the species to be spawned. In further studies a combination of photothermal manipulation following guidelines successfully used by Benetti [23] and Benetti et al. [39] as well has spawning peptide injection should be used to ensure sexual maturity and prime fish for successful spawn. The combining of both hormone injection and intensive photothermal manipulation can prime fish for spawning in multiple effective ways. The use of sGnRHa has been proven to effectively induce ovulation in early stage fish and has been effective at producing successful spawns in multiple species of fish [14,33-36]. Environmental manipulation integrates natural physiological functions, such as spawning, with natural environmental cycles thus priming fish for favorable spawning conditions. Water temperature has been demonstrated as the key environmental manipulation to induce off-season cobia spawning [24]. The key to successful spawning of cobia was a combination of hormonal injection that progressed fish that may have been in early stages of ovulation, as well as the photothermal manipulation that provided the proper environmental cues for successful spawning.
Conclusion
The induction of ovulation through hormone injection in sexually mature adult cobia was successful. The lack of fertilized eggs produced in this trial indicated that environmental conditioning as well as hormone injections may prove to be more successful. Also, the complications during this study (i.e., the presence of lesions created by numerous hormone implant injections required to achieve the desired dosage) will require further research for hormone injection.
To read more about this article...Open access Journal of Biology & Life Sciences
Please follow the URL to access more information about this article
To know more about our Journals...Iris Publishers





No comments:
Post a Comment