Authored by Carlos Landa Solís*,
Abstract
This review is focused the evolution of mesenchymal stem cells (MSCs) and their clinical applications in rheumatology. MSCs have significant properties immunomodulatory by suppressing T- and B-cell proliferation, dampening the generation of mature myeloid dendritic cells, and inhibiting the proliferation, cytokine production and cytotoxic activity of natural killer cells, and they have the capacity to engulf apoptotic cells. This work addresses the results in vitro and in vivo research on the current clinical applications of MSCs in rheumatology, as well as surface markers, cell culture techniques, regenerative properties, and immunomodulatory mechanisms of MSCs, as well as the practical limitations of the last nineteen years (2002 to 2021). MSCs have many clinical applications as well as isolation sources, and with very varied clinics results. Undoubtedly there is still much to discover about their clinical applications and the management of autoimmune diseases, but MSCs continue to represent valuable source for developing of new therapies for the treatment of rheumatologic diseases.
Keywords: Mesenchymal stem cell, Properties immunomodulatory, Rheumatology
Introduction
At the beginning of present century, the first works were reported that related to mesenchymal stem cells with some possible application in rheumatology. These new strategies began with the isolation and characterization of bone marrow multipotential mesenchymal progenitor cells BM-MSCs, using a positive selection of +CD45 (low) cells. The results in these studies showed that the BM-MSCs could be implications for defining the physiologic roles of MSCs in arthritis, bone diseases, and joint regeneration [1]. Continuing with this trend, the first reports appeared where other sources of MSCs with possible applications in rheumatology with special emphasis on the treatment of osteoarthritis (OA) were evaluated,[2] like membrane and liquid synovial,[3] at the same time are incorporated the use of hyaluronic acid scaffold,[4] and growth factors such as BMP2, to promote the differentiation of MSCs to chondrocytes with the subsequent repair of the surface of the articular cartilage[5]. After, two new sources of MSCs were evaluated, the human placenta and cord blood, in both cases, the aim was continued to experiment with joint repair strategies [6, 7]. The advent of all these new of cellular sources of MSCs in rheumatology considerably changed the evolution and prognosis of chronic inflammatory arthritis [8]. Which led to the study of the immunomodulatory properties of MSCs,[9] For the first time, was showed that MSCs exert immunosuppressive activities by suppressing T- and B-cell proliferation, dampening the generation of mature myeloid dendritic cells, and inhibiting the proliferation, cytokine production and cytotoxic activity of natural killer cells, [10, 11] and it was demonstrate that human MSC migrate upon stimulation with CXCL8 but not CCL2 [12]. To compare whether BM-MSCs are similar in patients with some autoimmune disease and healthy donors, BM-MSCs were isolated from patients with systemic lupus erythematosus (SLE) and normal controls. MSCs from SLE patients and normal controls were infused into ICR mice (Tac: Icr: Ha strain) after high-dose chemotherapy, with no adverse events in either group. Recovery of white blood cells, hemoglobin, and platelets was faster (P <0.05) compared to the group without MSC infusion. It was concluded that MSCs in SLE patients present abnormalities compared to those found in normal control. MSCs in SLE patients may play an important role in the pathogenesis of SLE [13].
At this point, It was determined that MSCs they home to inflamed tissue and exert an anti-inflammatory paracrine effect,[14] and the researchers were in an exploratory phase for the treatment of inflammatory arthritis,[15] and with more focused in acute autoimmune disease including SLE. In a case report about MSCs transplantation for diffuse alveolar hemorrhage in SLE, it was found that after transplanting with MSCs isolated from umbilical cord (UC-MSCs, infusion of 8x107 cells), the patient showed dramatic improvements in her clinical condition, oxygenation level, radiographic and hematological status, the patient was discharged from hospital approximately 5 weeks after undergoing transplantation [16]. Later, the allogeneic MSCs transplantation therapy for SLE was continued using UC-MSCs,[17] plus BM-MSCs, obtaining similar results [18-21]. On the other hand, MSCs in patients diagnosed with myelodysplastic syndromes or multiple myeloma showed abnormalities, which could play a role in the physiopathology of the disease. and patients with immune thrombocytopenic purpura, MSCs have a reduced proliferative capacity and a lower inhibitory effect on T-cell proliferation compared with MSC from normal control [22]. Other applications that were explored up to that point using MSCs were: treatment of therapy resistant graft versus host disease, Crohn’s disease and organ transplantation [20, 23-25]. In 2019 year ,was reported that the human UC-MSCs possessed the ability to engulf apoptotic cells (ACs). The MSC exposed to ACs (AC-MSC) increased MSCmediated suppression of CD4+ T cell proliferation compared to MSCs alone. Mechanistically, ACs stimulated MSCs to express COX2 and consequently produced PGE2 that inhibited T cell responses. NF-κB signalling pathway mediated the activation of COX2/PGE2 in AC-MSCs. Importantly, in patients with SLE, the plasma PGEM levels increased significantly in those with reduced apoptotic mononuclear cells in peripheral blood after MSC transplantation [26].
Ten years ago, the research works reported up to that point focused on the biological properties of MSCs, including their immunoregulatory characteristics, differentiation capacity and trophic potential, as well the relevance of delivery of antiinflammatory factors or immunomodulatory MSC-based therapies for rheumatic diseases like rheumatoid arthritis (RA) [27-29]. In this way, the first results of MSCs therapy for knee osteoarthritis were reported, with encouraging, but not excellent. with the observation that the Improvement of the technique may improve the results [30]. Also, advances in the treatment of SLE were reported, it was demonstrated that single MSCs transplantation at the dose of one million MSCs per kilogram of body weight was sufficient to induce disease remission for refractory SLE patients,[31] and the UCMSCs therapy was extensive to SLE patients with diffuse alveolar hemorrhage (DAH), with results that suggest that UC-MSCs therapy results in amelioration of oxygen saturation as well as hematological and serological changes, which revealed that UC-MSCs therapy could be applied as a salvage strategy for DAH patients [32].
After a 4-year follow-up after receiving allogenic therapy with MSCs, it was reported the induction of clinical remission, reverse hematological aberration with refractory cytopenia and improvement in organ dysfunction in drug-resistant SLE patients [33, 34]. in a similar study with lupus nephritis (LN) patient’s refractory to conventional therapy, it was reported no transplantation-related adverse event was observed and the Allogeneic MSC therapy resulted in renal remission for active LN patients within 12-month visit, confirming its use as a potential therapy for refractory LN [35]. One of the mechanisms reported to explain these improvements was that immune microenvironment in SLE patients can significantly stimulate the TGF-β1 expression on allogenic MSCs therapy and plays an important role in the upregulation of regulatory T cells in patients [36]. On the other hand, has been reported that autologous MSCs from lupus patients are not effective in treating disease. Furthermore, standard in vitro assays for MSC licensing are not predictive of in vivo efficacy, whereas inhibiting B cell proliferation appears to differentiate effective MSCs from ineffective MSCs [37]. Three years later it was reported that higher baseline levels of IFN-γ might predict a good response to MSC therapy for active lupus patients, which will help to choose suitable patients for clinical transplantation [38].
For exploring of UC-MSCs therapy in patients with active rheumatoid arthritis has been analyzed the treatment with diseasemodifying anti-rheumatic drugs plus UC-MSCs, this combination showed that may provide safe, significant, and persistent clinical benefits for patients with active RA [39]. It has also been shown that UC-MSCs therapy inhibits the expression of Cadherin-11 (CDH11) by fibroblast-like synoviocytes (FLS) in RA patients, and this mechanism might be targeted to ameliorate arthritis [40].Also, in circulating T follicular helper (cTfh) cells in primary Sjögren’s syndrome patients (pSS) and RA patients, exposed to UC-MSCs therapy, it has been observed an inhibitory effect of UC-MSCs on the differentiation of cTfh cells via the secretion of indoleamine 2,3-dioxygenase (IDO), and soluble factors secreted by activated CD4(+) T cells might contribute to IDO secretion by UC-MSCs,[41] these findings reveal a suppressive function of MSCs in cTfh cells [42]. Therefore, the modulation of MSC homing may allow targeted delivery of systemically administered MSCs to damaged articular cartilage, where they can suppress immune-mediated cartilage destruction and contribute to cartilage repair via a combination of chondrogenic differentiation and paracrine stimulation of intrinsic residual repair [43]. Three years later, the UC-MSC therapy continued to be studied in RA patients in a phase Ia clinical trial, where patients received a single intravenous infusion of 2.5×107, 5×107, or 1×108 cells of UC-MSCs for 30 minutes, three patients in each cluster, with an increment of cell numbers when there was no dose-limited adverse event. There was no major toxicity in all clusters up to 4 weeks after the infusion. Reduced levels of IL-1β, IL- 6, IL-8, and TNF-α at 24 hours were observed in the cluster infused with 1×108 MSCs. This study UC-MSCs infusion trial for established RA patients revealed no short-term safety concerns.[44] at the same time other research group reported similar safety analysis in patients with autoimmune disease that receiving allogeneic UCMSCs therapy a long-term, but in more diseases like: SLE, Sjögren’s syndrome, and systemic sclerosis, with an incidence of adverse events, whether infections or malignancies, are acceptable in these patients [45].
New strategies have been proposed for the treatment of refractory lupus nephritis and leukopenia, a case of autologous hematopoietic stem cell (HSC) transplantation combined with MSCs in a 25 year-old SLE patient with multiple life-threatening complications and refractory to conventional was reported cyclophosphamide (CYC) therapy, the findings suggest that the combined transplantation of HSCs and MSCs may reset the adaptive immune system to re-establish self-tolerance in SLE. A 36-month follow-up showed that the clinical symptoms remained in remission, and the combined transplantation of HSCs and MSCs may be a novel and effective therapy for refractory SLE [46]. At the same time by other research team, it was evaluated the osteoblastic potential of infrapatellar fat pad-MSCs (IFP-MSCs) cells from rheumatoid RA and OA patients, they concluded that IFP-MSCs from RA have comparable or slightly stronger osteogenic potential than IFP-MSCs from OA, these findings indicate that IFP-MSCs have the therapeutic potential for the development of new therapeutic strategies in both RA and OA pathologies [47]. At the same time to finish the 2015 year, was reported the evaluation of BM-MSCs therapy for knee osteoarthritis 5 years follow-up in three patients with advanced stage of OA, they observed that for the advanced stage of OA in the patients at 5 years continued its progression toward aggravation to disease. Due to these findings the authors recommended the use of therapy with BM-MSCs in early stages of OA for better long-term outcomes [48].
At the beginning of 2016, it was evaluated in 12 patients with follow-up for one year, the therapy with Autologous adiposederived stromal vascular fraction (ADSVF-MSCs), The results of this research were very promising, characterized mainly by a significant improvement of finger oedema, skin sclerosis, motion, and strength of the hands and of the vascular suppression score was also noted, the reduction in hand pain approached statistical significance (P = 0.052). The questionnaire revealed a benefit in daily activities, housework, and social activities, with the extension of all this benefits in the patients for one year [49]. In the following year, it was reported the effect of combined plasmapheresis (PE) and allogeneic MSC therapy on systemic sclerosis (SSc), obtaining apparent good results owing to feasible treatment associated with possible clinical benefit for SSc patients at one year of follow-up [50]. In a subsequent randomized placebo-controlled doubleblind trial to assess the safety of intramuscular administration of allogeneic MSC for digital ulcers in SSc. The patients received eight intramuscular injections with either placebo or 50x106 MSCs. Follow-up visits will be scheduled at 48 hours and 2-, 4-, 8-, 12-, 24- and 52-weeks post-treatment. If the results confirm safety, feasibility, and potential efficacy of this therapy [51].
In early 2018, repeated-dose adipose-MSCs (haMSCs) were evaluated in patients with a definite diagnosis of knee OA (between ≥2 to ≥4 grade), 18 patients were enrolled and divided into three dose groups: the low-dose, mid- dose and high-dose group (1×107, 2× 107 and 5×107 cells, respectively), provided three injections and followed up for 96 weeks. The results suggested that intraarticular injections of haMSCs improved the pain, the improvement was superior in a dosage of 5×107 cells combined with repeated injections, and the authors were cautiously optimistic regarding the articular injection of haMSCs as a putative treatment for knee OA [52]. In the next year in another study, was evaluated therapy with BM-MSC in refractory juvenile idiopathic arthritis (JIA) patients, using an intravenous infusions of allogeneic BM-MSC (2 million/kg cells), after the therapy no acute infusion reactions were observed and a lower post-treatment than pre-treatment incidence in adverse events was found, and it is recommended to continue with the drug immunosuppressant treatment to send the macrophage activation syndrome[53] Recently in 2020, was carried out a phase I pilot study, where the patients with symptomatic, bilateral knee osteoarthritis were randomized to three treatment groups (low dose, 1×107 cells; medium dose, 2×107 cells; high dose, 5×107 haMSC). All patients received two bilateral intra-articular injections: week 0 (baseline) and week 3, the follow-up was carried out by 48 weeks. Adverse events were transient, including mild to moderate pain and swelling of injection site. Improvements from baseline were measured in the secondary end points. MRI assessments showed slight improvements in the low-dose group and, Safety and improvements in pain and function after intra-articular injections of allogeneic human adipose-derived mesenchymal progenitor cells into arthritic patients was demonstrated [54].
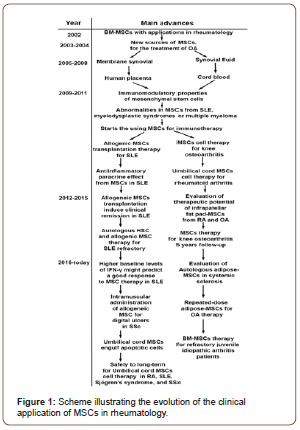
Conclusion
To date there is enough evidence to support the use of MSCs in the autoimmune diseases, like RA, SLE, myelodysplastic syndromes or multiple myeloma, and in other for maintain joint health such as OA.
Undoubtedly one of the fields in which the most progress has been made is the treatment of systemic lupus erythematosus, where UC-MSC have proven to be very useful and have even led patients to enter into remission in cases where patients presenting refractory to conventional therapy.[33, 35, 42] On the other hand, in the treatment of osteoarthritis, it has been observed to obtain better results when therapy with MSCs is applied in early stages of the disease since it has been reported that when therapy with BM-MSC advanced stage of OA is applied, to long term all benefits of treatment are lost [Figure 1] [30, 48].
Therefore, and according to current trends of last reported studies, the community of clinical and basic researchers need together to propose large prospective controlled trials, for the development of new therapies that help to the best application of MSCs in the treatment of rheumatic diseases.
In this review, I show the points that were most important to me in the development of MSC therapies at today and the challenges that still need to be solved in the future.
To read more about this article...Open access Journal of Rheumatology & Arthritis Research
Please follow the URL to access more information about this article
To know more about our Journals...Iris Publishers





No comments:
Post a Comment