Authored by Mosaab Akram Elshaer*,
Introduction
Factors related to cardiac surgery that contribute to myocardial damage include the duration of cross clamping and cardiopulmonary bypass; potential occlusion of a graft; the nature, temperature, and adequacy of the cardioplegia; the use of cardiopulmonary bypass itself (owing to activation of platelets, complement and cytokines); direct trauma to the heart; coronary artery or venous graft embolism; and other complications of the procedure. Some damage is unavoidable. The relevant clinical issue is to define whether the degree of myocardial damage is “clinically significant.” Biomarkers cannot determine the mechanism of injury However, irrespective of the mechanism, the higher the value after surgery, the greater the damage and the worse the prognosis [1].
Traditionally the diagnosis of cardiac events after open heart surgery has relied on the use of electrocardiography, echocardiography, and enzymatic markers of myocardial injury. Although the presence of new significant contiguous Q waves on serial ECGs is 90% specific for cardiac damage, the sensitivity in this setting is low. Echocardiography is of limited use in this setting, in part because there may be myocardial stunning not associated with lasting injury. Furthermore, previous wall motion abnormalities and difficulty in obtaining optimal studies in all patients in the intensive care unit setting makes interpretation difficult. Although enzyme markers of myocardial damage have traditionally been used to aid in the detection of myocardial infarction, their use in the surgical setting has been limited by their presence in skeletal muscle [2].
Surgically induced myocardial ischemia secondary to aortic cross clamping, results from the attenuation or cessation of coronary blood flow such that oxygen delivery to the myocardium is insufficient to meet basal myocardial requirements to preserve cellular membrane stability and viability (Levitsky S, 2016). By maintaining native coronary blood flow in on-pump beating heart surgery and comparing with OPCAB strategy pumprelated effects on myocardial injury and cardiac dysfunction could be specifically differentiated from ischemia/reperfusionrelated consequences of surgical coronary revascularization [3]. Myocardial cellular damage associated with cardiac surgery can be caused by different mechanisms, including direct myocardial trauma by surgical manipulations, these aetiologias of myocardial damage can all result in myocardial necrosis and therefore lead to the elevations of cardiac biomarkers and enzymes. (Matthias Thielmann, et al, 2015).
The objective is to compare postoperative cardiac troponin I (cTnI) release and the thresholds of cTnI that predict adverse outcome after coronary artery bypass graft (CABG), after valve surgery, and after combined cardiac surgery (Fellahi et al 2017).
Aim of the work
Evolution of the Perioperative Myocardial Injury Characterization: Incorporating the Perioperative Scenario and demonstration of different factors that lead to myocardial injury.
Discussion
Effects of Ischemia on Myocardial Cell structure & function
Liberation of free fatty acids in lipolysis is stimulated in myocardial ischemia by increased circulating catecholamines, but fatty acid oxidation and tricarboxylic acid cycle are inhibited. Finally, the accumulation of protons, lactate and reduced form of nicotinamide adenine dinucleotide (NADH) lead to inhibition of glycolysis and anaerobic energy production through it (Stanley et al, 2017).
Finally, within hours myocardial contracture is developed, which microscopically is characterized by contraction band necrosis, distortion of Z-bands and breakdown of myofibrils. Cell death and tissue necrosis finally develop, followed by inflammation and fibrosis (Peuhkurinen KJ, 2020).
Reperfusion Injury
Early reperfusion is an absolute prerequisite for the survival of ischemic tissue. Reperfusion, although ultimately necessary for recovery is, however, considered a double-edged sword, and can lead to worsening of tissue injury by various mechanisms [4].
A few mechanisms have been proposed to mediate reperfusion injury. These include cellular calcium loading; the occurrence of a no reflow phenomenon due to cell swelling, impaired vascular relaxation or the formation of white cell plugs; and perhaps most importantly the formation of oxygen radicals. While low levels of oxygen radicals and oxidants are normally formed in cells and play important roles in cellular homeostasis, mitosis, differentiation, and signaling. (Irani K. et al, 2007) (Figures 1,2).
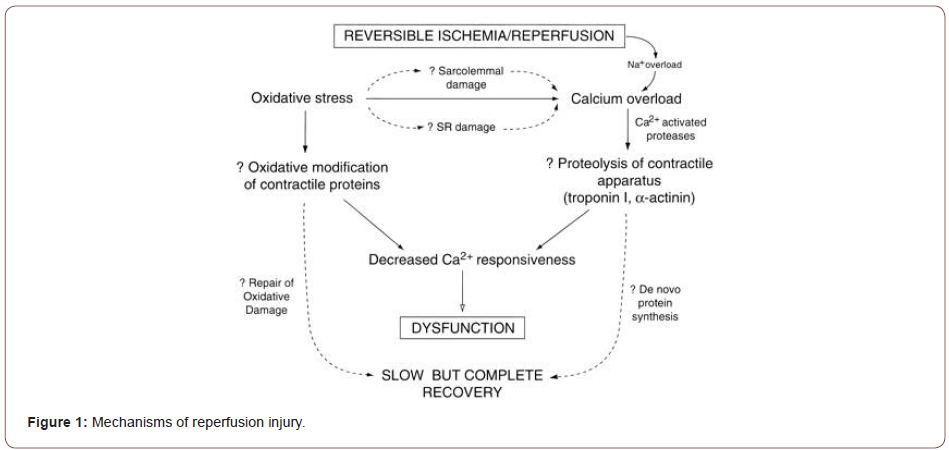
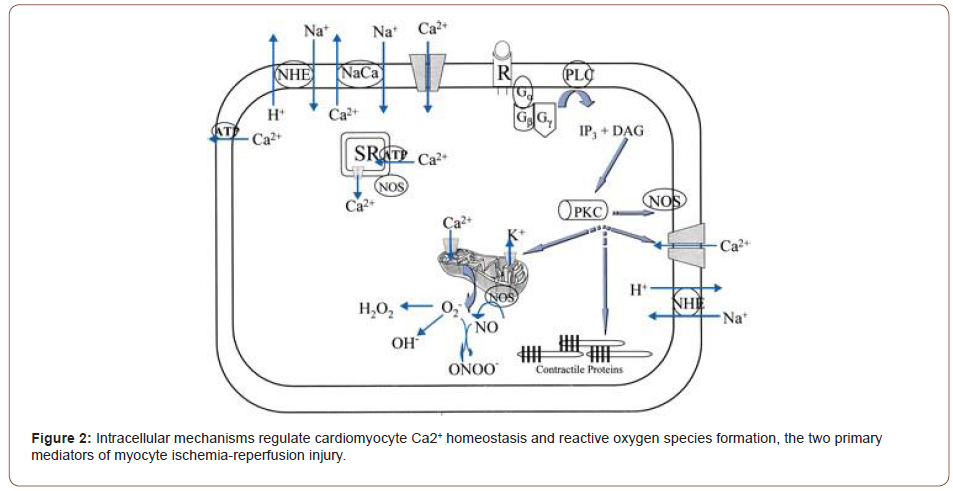
Oxygen free radicals
The term “free radical” is used to define an atom or molecule that can exist independently with one or more unpaired electrons. By virtue of their unpaired electron, free radicals are typically unstable, highly reactive, and short-lived. Oxygen-derived free radicals and oxidants (reactive oxygen species, ROS) are formed continuously in small amounts during the normal metabolism of cells and are normally inactivated by endogenous scavenging mechanisms (Zweier J & Talukder MA, 2006).
Free radicals are generated by one electron reduction or oxidation of molecules creating an unpaired electron. In normal mitochondrial oxidative phosphorylation, O2 is reduced by four electrons to form H2O. The energy derived from this reduction of O2 serves to meet the energy demands of the cell. Paradoxically, it is also the actual process of oxygen reduction that leads to the formation of oxygen free radicals. Incomplete reduction of O2 leads to the generation of superoxide anion (O2), hydrogen peroxide (H2O2), and hydroxyl radical (OH). O2 is unstable with a lifetime of milliseconds at neutral pH, and in aqueous solution it spontaneously reacts or dismutates to yield H2O2 and O2. The OH is an extremely reactive and short-lived free radical produced in biological systems. In the Haber-Weiss reaction, O2 and two OH are formed when O2 reacts spontaneously with H2O2. In the Fenton reaction (also known as the iron-catalyzed Haber-Weiss reaction), reduction or oxidation of a trace metal in the presence O2 and H2O2 gives rise to OH (Zweier J & Talukder MA, 2006) (Figure 3).
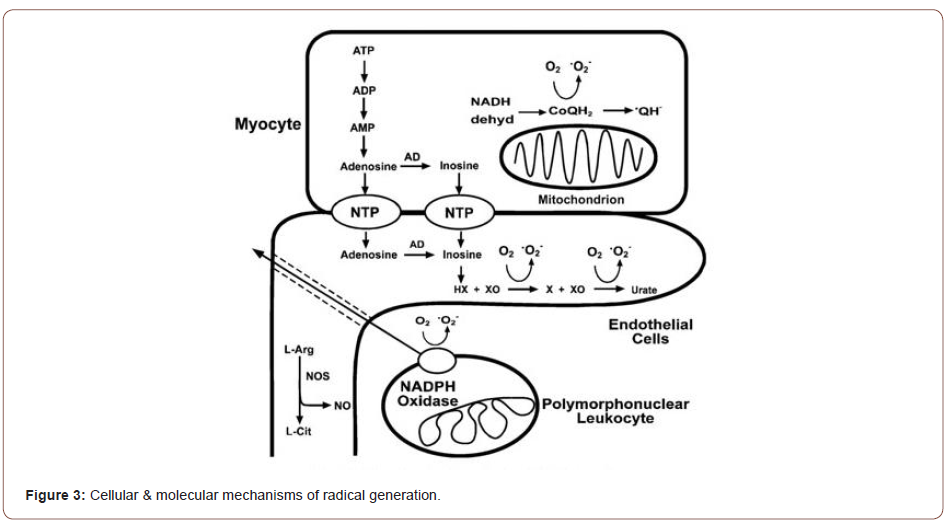
Following ischemia and reperfusion radical formation is greatly increased triggering cellular injury (Hano O et al, 2014). Over the last three decades, it has become clear that oxidants and free radicals are generated in the ischemic and reperfused heart and are important mediators of post-ischemic injury. Both oxygen radical and Nitric oxide (NO), formation is greatly increased through a series of interacting enzymes and cellular pathways. In the setting of myocardial ischemia and reperfusion with the marked increases in oxygen radical and NO generation, this balance is disrupted resulting in oxidative injury (Zweier J & Talukder MA, 2016).
Calcium Overload
Restoration of intracellular pH at the onset of reperfusion via Na+-H+ exchange contributes to intracellular Ca2+ overload via reversed Na+-Ca2+ exchange [5].
Myocyte calcium levels have been observed to increase upon reperfusion of ischemic myocardium. Damage to the cell membrane and sarcoplasmic reticulum, possibly by free radical species, results in a net increase in the concentration of intracellular calcium. This results in relative insensitivity of contractile elements to calcium with subsequent depression of myocyte contractility. This phenomenon is known as the “calcium paradox” (Gross 2018, Park, 2019).
Types of Reperfusion Injury
1- Myocardial Stunning
When myocardial ischemia is limited to periods of less than approximately 20 minutes, reperfusion of the affected tissue will lead to recovery following transient changes in cellular structure, function, and metabolism. These changes are manifested
Despite much improvement in surgical procedures the benefits of cardiac surgery are delayed due to postoperative left ventricular dysfunction. It is believed that the postoperative dysfunction is due to global myocardial stunning since the heart is rendered globally ischemic during the surgical procedure (Bolli, 2019). As a consequence of stunning the benefits of reperfusion therapy, cardiac surgery and transplantation are delayed. Furthermore, it may contribute to both morbidity and mortality by causing the incidence and severity of postoperative complications to significantly increase in high-risk patients (Janith Wickramaratna 1998) (Figure 4).
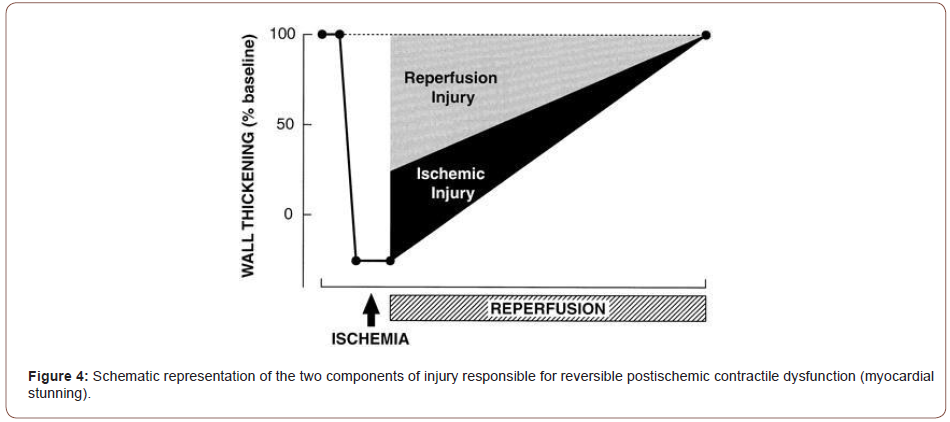
2- Apoptosis vs. Necrosis
Necrosis and apoptosis are two forms of cell death in the myocardium that have been associated with ischemia and reperfusion. Although it has been well documented that necrosis, as a major form of myocyte cell death, rapidly leads to a destruction of a large group of cells after myocardial ischemia and reperfusion, the induction of apoptosis in myocardium, primarily triggered during reperfusion, may independently contribute to the extension of cell death (i.e., infarction) in a dynamic manner (Zhao Z & Vinten- Johansen J, 2012).
Collective evidence from experimental and clinical studies has demonstrated that cardiomyocyte cell death following ischemia and/or reperfusion is now known to involve apoptosis, oncosis, autophagy, (and necrosis) in ischemic cardiac conditions. These conditions are thought to lead at later stages to necrosis [6,7].
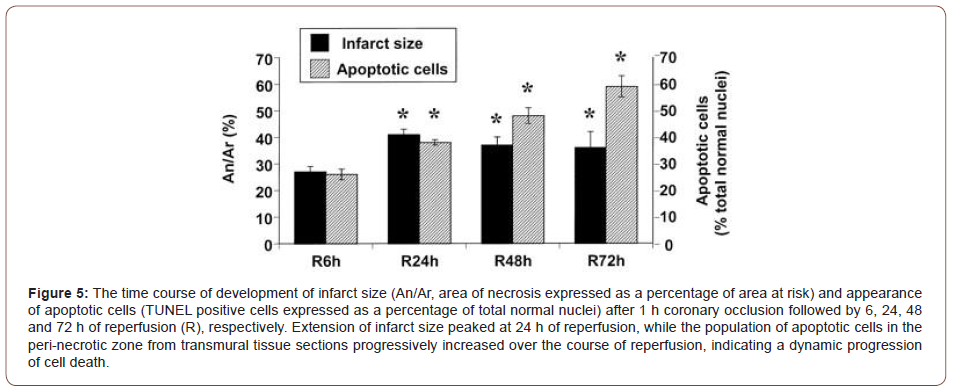
Data demonstrates that programmed cell death is evident early after open heart surgery and correlates with declining cardiac contractility. Concluding that, apoptosis may be an important mechanism in postoperative myocardial dysfunction [8] (Figure 5).
Conclusion
In the postnatal period the precise investigation of the child gives the mother the certainty that her child has the best chances to develop. The medical team member must know each other before the child is born even if they are dispersed but they can reach each other via internet and mailing and other electronic facilities, so the diagnosis is complete, and each specialist is tied to the other ones. Time sparing and always reassurance of the mother that the child is under permanent surveillance and the access of the child’s family to all the specialists prevent anxiety, doubt and fear so the postpartum depression is prevented. Always a better outcome of a child means more security and more happiness in each family.
Mechanisms of cell death
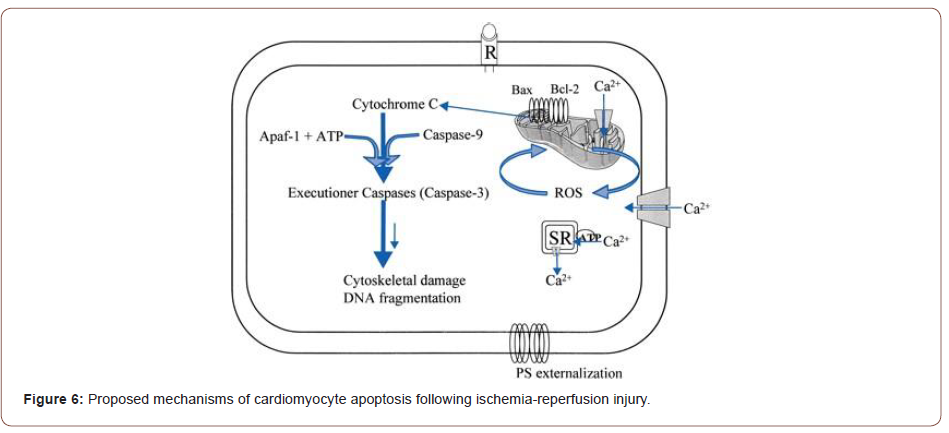
(Figure 6)The ultimate fate of a cardiac cell, whether it lives or dies, seems to be a delicate balance of the factors promoting cell death and those factors promoting cell survival. There has been a recent explosion of information regarding the analysis of these factors, and we were able to touch upon select pathways due to restrictions in space.
Although an explosion of data has emerged from the growing field of cardiomyocyte cell death in ischemia, many questions are left unanswered. Even the rates of apoptosis and cell death vary widely from study to study. Differences in experimental data could be the result of the use of different models (occlusion vs constriction), ischemia and reperfusion time lengths, species, inhibitors, transgenic models, and the methods used in detection of cell death (Elsasser et al, 2019).
An important factor in the initiation of necrosis or apoptosis is the opening of a nonspecific pore within the inner membrane of the mitochondria, the mitochondrial permeability pore (MPTP). Current data support the fact that stress stimuli induce the pore to open, releasing pro-apoptotic factors into the cytosol (McFalls et al, 2013) (Figures 7,8).
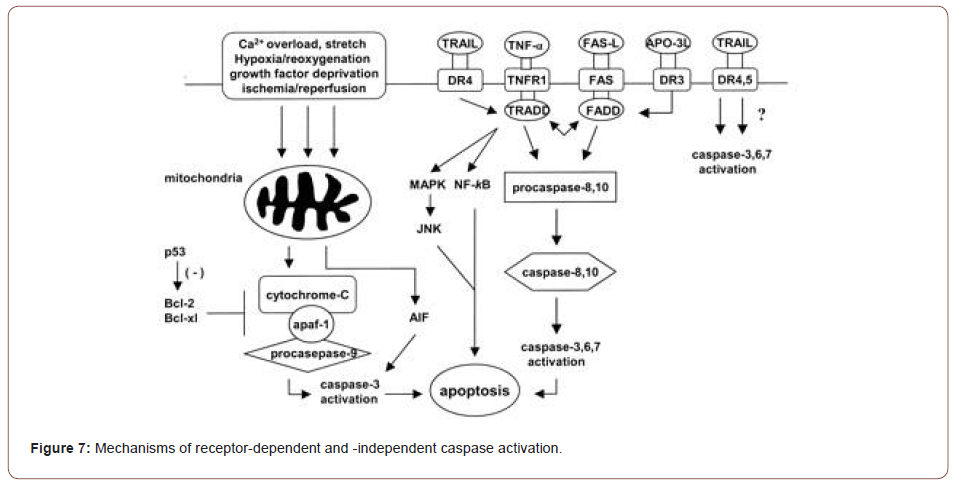
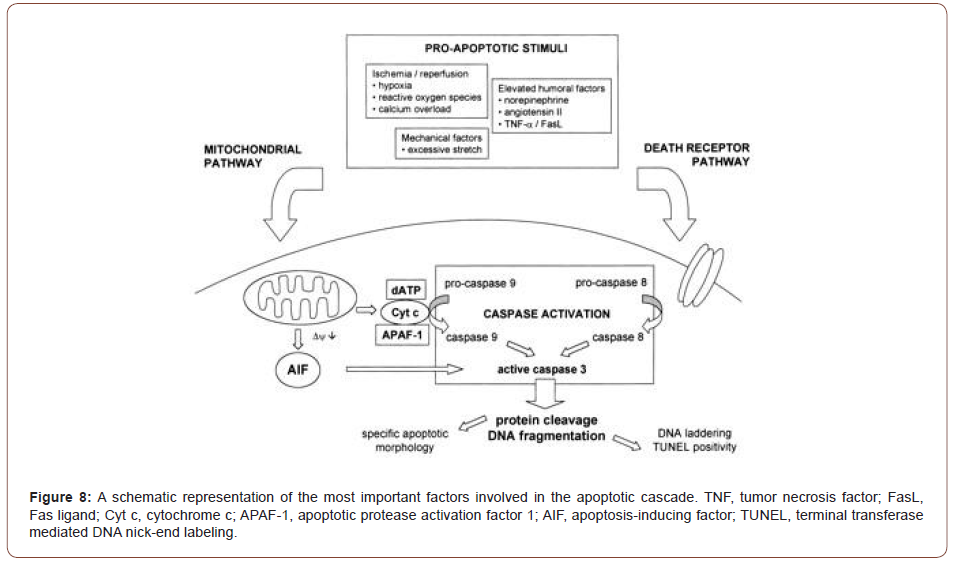
Immunohistochemical analysis of ischemic/reperfused left ventricle showed caspase-3 levels were substantially elevated and localized in the ischemic/reperfused region, and that caspase-3 colocalized to TUNEL positive myocytes in an in vivo rat model [9].
On the other hand, Okamura et al. examined the effect of caspase inhibitors on myocardial infarct size and CM DNA fragmentation in ischemia-reperfused rat hearts. Caspase inhibitors were able to inhibit myocyte DNA fragmentation and caspase activation; however, there was no significant reduction of the infarct size in ischemiare perfused rat hearts. (Okamura et al, 2010) (Figure 9).
The STAT family of transcription factors has been shown to activate caspases. Ischemia/reperfusion was shown to induce STAT-1 activation and caspase-1 processing in ventricular myocytes in the intact heart ex vivo. STAT-1-transfected cells were more susceptible to ischemia-induced cell death than control transfected cells. A possible mechanism was shown where STAT-1 may activate the promoter of the pro-apoptotic caspase-1 gene in cardiomyocytes during ischemia. Antisense STAT-1 vectors reduced both ischemia- and over expressed STAT-1-induced cell death in cardiac cells. These results suggest that STAT-1 plays a critical role in the regulation of ischemia/reperfusion-induced apoptosis in cardiac cells, acting at least in part via a caspase-1 activationdependent pathway (Stephanou et al., 2018).
Necrosis is a general term used to describe another mode of cell death. Using the term necrosis is problematic since dead cells are so severely degraded at the final stages that they cannot be morphologically determined whether they died via apoptosis or necrosis. (Manjno and Joris, 2015). The lower LVEF have higher myocardial cell apoptosis. This clearly indicates that left ventricular dysfunction is closely associated with the presence of myocardial cell apoptosis (Kazuhiko Doi, et al 2014).
Graft Occlusion
Cardiac death, myocardial infarction and heart failure were more frequent in patients with graft occlusion, (Vavlukis M, et al. 2016). The most common graft-related reasons for PMI are graft occlusion, graft kinking or overstretching, subtotal anastomotic stenosis, or graft spasm (Mangano DT, 2012). Low graft flow rate is one of the most important factors that increase the likelihood of early graft occlusion. Circumstances that cause a low graft flow rate are small luminal size (< 1.5 mm) of the grafted artery and decreased distal runoff due to severe disease in the recipient artery. The specific artery grafted is also important: The occlusion rate of a saphenous vein graft to the right coronary and circumflex arteries is higher than that for left anterior descending grafts. (Fitz Gibbon GM, Leach AJ, Kafka HP, Keon WJ 1991).
The use of the internal mammary artery also complicates myocardial protection at reoperation. Retrograde cardioplegia is often necessary to reach and protect areas of myocardium perfused by a patent internal mammary artery. Such areas would otherwise remain warm and prone to ischemic injury (Nwasokwa ON, 1995).
Perioperative venous graft failure after off-pump CABG procedures is chiefly determined by the two factors of graft endothelial damage and patient hyper coagulability (including resistance to anti platelet therapies) (Frazier A, et al 2005) [10-14].
Summary
• Although perioperative myocardial injury after cardiac surgery is an accepted clinical outcome responsible for a several fold increase in morbidity and mortality, there is currently no effective clinical gold standard for its detection. Cardiac surgery, especially, is associated with the inherent risk of myocardial ischemia and myocardial infarction; and consequently, with postoperative heart failure. (Liakopoulos OJ, et al 2015).
• Both experimental and clinical studies have ascertained the role of several hormonal mediators, mitochondria, cardioplegia and CPB temperature, apoptosis and even genetic modulators of damage. However, the correlations between these factors in vivo and post-surgery outcome and prognosis have not yet been systematically investigated. (Amedeo Anselmi et al 2014) [10].
• While the etiology of post ischemic myocardial dysfunction after cardiac surgery is multi factorial, three basic types of injury occur during heart surgery: myocardial stunning, apoptosis, and MI. (Elsasser A et al 2018).
• Myocardial cellular damage associated with cardiac surgery can be caused by different mechanisms, including direct myocardial trauma by surgical manipulations, focal damage caused by inadequate cardioplegic perfusion, and inadequate myocardial protection. Myocardial damage can also be induced by coronary artery microembolization and result in focal inflammation and regional contractile myocardial dysfunction. These aetiologies of myocardial damage can all result in myocardial necrosis and therefore lead to the elevations of cardiac biomarkers and enzymes. (Thielmann M, et al, 2015) [14-16].
To read more about this article....Open access Journal of Biomedical Engineering & Biotechnology
Please follow the URL to access more information about this article
To know more about our Journals...Iris Publishers





No comments:
Post a Comment