Authored by Mizaton Hazizul Hasan*,
Abstract
Intsia palembanica (Fabaceae) is a tropical heartwood indigenous to Malaysia and countries of similar climates. The tree is commercially used for its timber. The aim of this study was to examine the antioxidant capacity of I. palembanica, reputedly rich in polyphenols, with a view to exploiting its sawdust as a source of antioxidants for the pharmaceutical and/or nutraceutical industries. The ethyl acetate fraction of I. palembanica was assessed for total phenolics and for antioxidant activity by several methods, viz, DPPH radical scavenging assay, ferrous chelating ability and inhibition of xanthine oxidase in purine metabolism. The results showed the ethyl acetate extract of I. palembanica to be a potent antioxidant, in vitro. Total phenolics in the extract was 668.2 ± 58.2 μg catechin equivalents/g. Median effective concentrations (EC50) for DPPH scavenging were 7.9±0.01 μg/mL and 4.5±0.02 μg/mL for the extract and standard antioxidant, trolox, respectively. EC50 for Fe2+ chelation was 19.95±2.37 mg/mL and 0.032±0.002 mg/mL for the extract and its comparator, EDTA. I. palembanica was very potent in inhibiting the production of uric acid with IC50 value of 6.3±2.5 μg/mL comparable to allopurinol which was 4.0±1.0 μg/mL. Median inhibitory concentrations (IC50) for inhibition of tertbutylhydroperoxide was 1.26 μg/mL, in comparison to the standard antioxidants, alpha-tocopherol acetate (IC50 = 1.58 μg/mL) and trolox (IC50 = 3.98 μg/mL). In summary, the ethyl acetate fraction of Intsia palembanica showed very potent antioxidant activity via various mechanisms. This holds promise for its use as a phytoantioxidant.
Keywords: Intsia palembanica; Antioxidant; Microsomal lipid peroxidation; DPPH; Fe2+; Xanthine oxidase
Introduction
Plants are playing an important role in maintaining human health and improving the quality of life. Since their ancestors, many people from developing countries have been using traditionally plant-derived products, especially from forests, to treat human and livestock diseases Rao et al. A few countries such as China, India, Thailand, Sri Lanka, Cuba, and a few others have sanctioned the official use of traditional of medicine in their healthcare programs Prajapati et al. [1]. Modern pharmacopoeia currently has about 25% of drugs obtained from plants and several others are synthetic analogues built on prototype compounds isolated from plants [2]. A study has been done on the Australian tropical forests on the pharmacological activities of 90 species of plants representing 19 families. 53 of the plant species examined indicated phytomedicinal potential for further investigation [3]. Most of the pharmacological effects of these plants are contributed to the presence of phenolic constituents which are broadly distributed in the plant kingdom. Many studies have found strong relationship between the presence of phenolic constituents (flavanoids, phenolic acids, diterpenes and tannins) in plants and their high antioxidant activities (Rice-Evans et al., 1995; Velioglu et al., 1998; Nakachi et al., 2000; Conforti et al., 2009). Some epidemiological studies have suggested an association between the consumption of phenolic-rich fruits and vegetables or beverages and the prevention of cardiovascular diseases and cancer [4-7]. These pathological conditions are connected with excessive free radical production that could also lead to various serious diseases such as neurodegenerative disorders, atherosclerosis, cataracts and inflammation (Aruoma, 1998; Liu and Stern, 1998; Babior and Andreoli 2000). Iron can induce lipid peroxidation by the Fenton reaction, and also increase lipid peroxidation by highly reactive hydroxyl radical generated from biological systems (Liochev, 1999). Numerous studies confirmed the ability of plant polyphenols to scavenge free radicals and chelate metal ions, established their potential act as chain-breaking antioxidants [8].
Intsia palembanica (Fabaceae or Leguminosae) is an indigenous heartwood which has been shown to be rich in polyphenols [9]. The leaf, root and bark of Intsia palembanica are used in traditional medicine for the treatment of urinary infections, rheumatoid arthritis and dysentry [10-12]. The potential value of Intsia palembanica as an antioxidant lies in its polyphenolic content, which include the stilbene, piceatannol and the flavonoids, myricetin and robinetin [9]. The presence of tannin, which is an astringent polyphenolic compound, and other polypenols such as flavonoids in some Chinese and Congolese medicinal plants have been reported to have significant effect in treating dysentery, diarrhea, bleedings and various skin inflictions [13,14]. It is well known that tannins and flavonoids have anti-inflammatory, antidiarrheic and wound healing properties [15-17]. Therefore, many studies have been carried out on medicinal plants to investigate the presence and activities of polyphenols and relate to their antioxidant activities to support their traditional claims [18-24]. This study was designed to determine the antioxidant potential of I. palembanica by studying its effects on different antioxidant assays. The abilities of I. palembanica fraction to scavenge DPPH radical (DPPH•) and chelate metal ions were examined. Inhibition of malondialdehyde (MDA) formation in hydroperoxide-initiated lipid peroxidation in rat liver microsomes was also identified.
Materials and Methods
Plant material
Wood sample was supplied by Forest Research Institute Malaysia, Kepong from its repository of timber wood samples. Confirmation of the identity was obtained from microscopical examination of a fragment of the sample.
Extraction and isolation
The extraction of the heartwood was adapted from [25]. The planks of Intsia palembanica were ground into powder and delipidated by hexane (maceration for 20 hours, lixiviation with fresh solvent for 4 hours). After removal of hexane, the samples were extracted by methanol (maceration for 20 hours, lixiviation with fresh solvent for 8 hours). The combined methanolic extracts were evaporated to dryness. The resulting residue was then partitioned between water and ethyl acetate. The ethyl acetate solution was dried under reduced pressure to yield the ethyl acetate fraction. Intsia palembanica ethyl acetate fraction was then subjected to a primary fractionation on Sephadex® LH-20 (load 1%) using a Büchi medium pressure liquid chromatography system, operated at 7 bars. A gradient mobile phase consisting of MeOH/H2O 50:50 to 100% MeOH were used. The separation was monitored by recording the ultraviolet (UV) absorbance at two wavelengths (283 and 340 nm). This pre-fractionation yielded 5 fractions.
Chemicals
Glutathione reductase, thiobarbituric acid, trichloroacetic acid, 1,1-diphenyl-2-picryl-hydrazil, EDTA, quercetin, trolox and ferrozine were obtained from Sigma (St. Louis, USA).
Animals
Male albino Sprague-Dawley rats (180-250 g) were obtained from the Universiti Kebangsaan Malaysia, Malaysia animal house. They were maintained on standard animal pellets and water ad libitum. Permission and approval for animal studies were obtained from the Animal Ethics Committee, Universiti Kebangsaan Malaysia. The rat was killed, and liver was excised for the preparation of liver microsomal system. A total number of 6 rats were used in this study.
Phytochemical screening
Extract (20 mg) was dissolved in 10 mL of methanol for 10 min and filtered. Filtrate (1000 μL) was transferred into two test tubes and a few drops of concentrated HCL were added. To test for flavonoids, the first tube was shaken for 2 min, and a piece of magnesium powder was then added. A positive result was indicated by a change in colour of the sample to red. The second tube was shaken for 2 min and a few drops of Mayer’s reagent were added. Formation of a yellow-cream precipitate showed presence of alkaloids. To detect presence of saponins in extract, 500 mg of extract was mixed with 5 mL distilled water in a test tube which was then shaken vigorously. Formation of a stable froth for at least 15 min indicated presence of saponins.
Determination of total phenolic content
Total phenolic content in the Intsia palembanica ethyl acetate fraction with Folin–Ciocalteu reagent, according to the method of Singleton & Rossi (1965) using catechin as a standard. Briefly, saturated sodium carbonate (0.5 mL) were added to 0.5 mL of the prepared sample and left for 15 min. Then, 0.5 mL Folin-Ciocalteu reagent (1:3 v/v diluted with water) were added and incubated for another 35 min. The absorbances of the samples were measured at 725 nm by spectrophotometer (Amersham 2100Pro, UK). All samples were analyzed in triplicates. The results were expressed as microgrammes of catechin equivalents per g dry weight extract by using an equation that was obtained from the standard catechin graph.
DPPH scavenging activity
The free radical scavenging activity of Intsia palembanica extract was measured by 1,1-diphenyl-2-picryl-hydrazil radical (DPPH•) using a modified method described by [26]. Briefly, 0.1 mM solution of DPPH• in ethanol was prepared. 10 mL of the Intsia palembanica fraction in methanol at different concentrations (0.01- 1 mg/mL) was added to 990 mL of the solution. The mixture was shaken vigorously and allowed to stand at room temperature for 10 min. Then, the spectrophotometric measurement was taken at absorbance 517 nm (Amersham 2100Pro, UK). Lower absorbance of the reaction mixture indicated higher free radical scavenging activity.
The percentage of DPPH• scavenging effect was calculated using the following equation:
DPPH• scavenging effect (%) = A1/A0 x 100
Where A0 was the absorbance of the control reaction and A1 was the absorbance in the presence of the standard sample or the I. palembanica fraction.
Ferrous ion chelating activity
Ferrous ion chelating (FIC) activity was measured by inhibition the formation of iron (II)-ferrozine complex after treatment with Fe2+ [27]. Briefly, the reaction mixture contained 500 μL samples or EDTA, 100 μL of 0.6 mM FeCl2 and 900 μL of methanol. Methanol was used as a blank. EDTA was used as a standard reference. Intsia palembanica was dissolved in methanol. The mixture was shaken vigorously and left at room temperature for 5 min. After that, 100 μL of 5 mM ferrozine in methanol was added, mixed and incubated for 10 mins at room temperature to complex the residual Fe2+. The purple colour solution which formed indicated the formation of ferrous-ferrozine complex and spectrophotometric measurements were carried out at 562 nm against the methanol blank.
The percentage of chelating agent will be calculated by using the following equation:
Chelating effect (%) = [(1- A1) / A0] x 100
Where A0 was the absorbance of the control reaction and A1 was the absorbance in the presence of the standard sample or the Intsia palembanica fraction. The chelating effect (%) was plotted against the log concentration of the sample in mg/mL of reaction mixture. The effective concentration that produced 50% scavenging effect value (EC50) was obtained from the graph (% chelating effect vs. log concentration).
Xanthine oxidase inhibitory assay
Xanthine oxidase inhibitory (XOI) activity was estimated by the formation of uric acid from xanthine–xanthine oxidase system under aerobic condition (Kong et al., 2000). Briefly, the reaction mixture containing 400 μl of 200 mM phosphate buffer (pH 7.5), 200 μl of 0.6 mM xanthine, 20 μl of sample solution, 180 μl of distilled water and 200 μl of 1 u xanthine oxidase enzyme were directly mixed in the cuvette. Samples were weighed and serial dilution was done to make six different concentrations which were 10, 1, 0.1, 0.01, 0.001 and 0.0001 mg/ml (final concentration in the cuvette). Allopurinol that was the standard was dissolved in the DMSO and Intsia palembanica was dissolved in methanol. After that, all working reagents and enzyme in cuvette were mixed thoroughly. The mixture was incubated for 1 minute at room temperature. The inhibitory activity of xanthine oxidase was assessed by measuring the absorption increment at 295 nm at room temperature which indicating the formation of uric acid from xanthine.
The inhibition of xanthine oxidase was measured as % of inhibition = (1 - β / α) x 100, where α is the change in absorbance per minute without the sample (ΔAblank with enzyme - ΔAblank without enzyme), and β is the change in absorbance per minute with the sample (ΔAtest with enzyme - ΔAtest without enzyme). The inhibitory effect (%) was plotted against the log concentration of test material in μg/mL of reaction mixture. From the graph, effective concentration that produces 50% inhibition effect value (IC50) was determined.
Liver microsomal system
Rat liver microsomes were prepared by tissue homogenization with 4 volumes of ice-cold 0.15 M KCl. Microsomal fractions were isolated in phosphate buffer, pH 7.4, by removal of the nuclear fraction at 3000 g for 10 min, removal of the mitochondrial fraction at 10,000 g for 30 min and sedimentation at 105,000 g for 60 min. Fractions were washed twice in the buffer and the microsome pellets were stored at -80° C for a maximum of one month.
Hydroperoxide-initiated lipid peroxidation
The inhibitory effect on lipid peroxidation was assessed by measuring TBARS by a modified method of [28], mainly constituted by MDA. Microsomal fractions were diluted with 0.05 M Tris-HCI, pH 7.4, containing KC1 (0.15 M). The final protein concentration in the incubation mixture was determined by the Lowry method and amounted to 0.75 mg/mL. The mixture was then preincubated in a shaking waterbath at 37°C for 10 min with different concentrations of the Intsia palembanica fraction. Lipid peroxidation was initiated with 3 mM tert-butyl-hydroperoxide. After incubating the samples for 10 min, 0.2 mL of the incubation mixture were taken out immediately into a test tube and 0.5 mL 20% trichloroacetic acid (TCA) was added to stop the lipid peroxidation reaction. 0.75 mL 0.67 % thiobarbituric acid (TBA) was added in the mixture followed by 10 min incubation in 95°C waterbath. 2 mL of n-butanol was added and vortexed vigourously for 3 min. After centrifugation at 3000 g for 10 min, the separated butanol layer was measured at Ex 515 nm, Em 553 nm using spectrofluorometer (Perkin-Elmer, UK). Inhibition of lipid peroxidation was expressed as a percentage, and the IC50 was obtained from the inhibition curve.
Statistical analysis
Data were expressed as means ± S.D. Statistical analysis was performed using Student’s t-test or by one-way analysis of variance, followed by post-hoc test for multiple comparisons of unpaired data. A value of p<0.05 was considered significant.
Results and Discussion
HPLC analysis of the extract
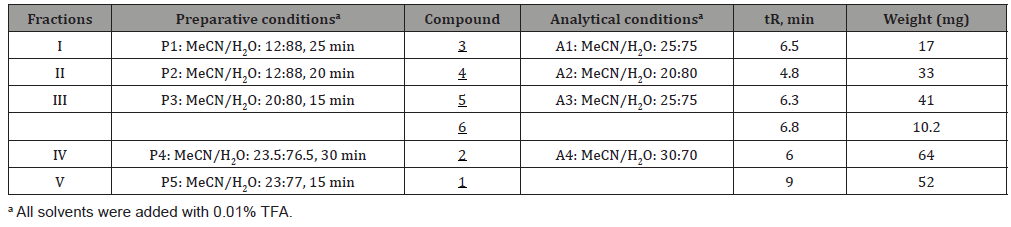
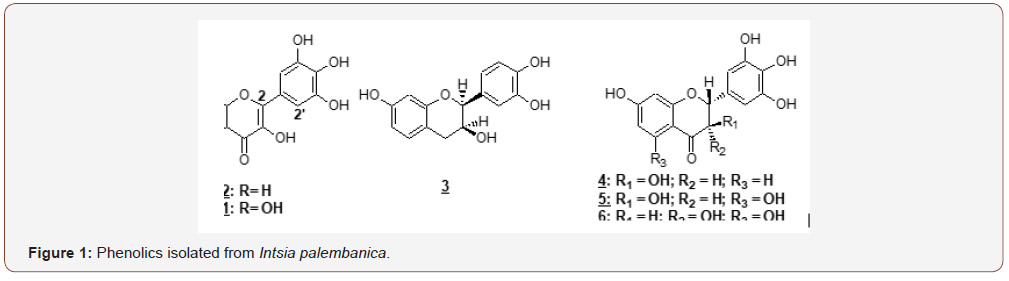
For the 5 fractions, methods P1 to P5 were respectively developed Table 1. The compounds are identified as follows. Fraction I gave compound 3, robinetinidol (tR = 23.5 min) after purification with prep HPLC, isocratically at 12% MeCN. Meanwhile, fraction II yielded dihydrorobinetin 4. Fraction III yielded two isomers of ampelopsin: 5, (2R, 3S)- and (2R, 3R)-ampelopsin 6 were found to be in ratio of 80:20 (tR = 9.7 and 11.1 min, respectively). These two compounds were examined by the same analytical HPLC procedure (MeCN: H2O = 25: 75). tR were found to be 6.7 and 6.3 min for (2R, 3S)- and (2R, 3R)-ampelopsin, respectively. Robinetin 1 was separated as a single constituent from fraction IV. Finally, myricetin 2 was eluted from fraction V. It was found out that the composition of I. palembanica was much more complex than it had been previously reported [9]. Robinetin and myricetin have been earlier isolated from I. palembanica, while compounds robinetinidol, dihydrorobinetin and the two isomers of ampelopsin [ (2R, 3S)- and (2R, 3R)-ampelopsin] are new compounds being identified from this species (Figure 1). These polyphenols have been found to possess anticancer, antimutagenic, antibacterial, antiobesity, antidiabetic activities [29-31] (Table 1).
Table 1: HPLC conditions for the fractions and the compounds from I. palembanica.
Phytochemical screening
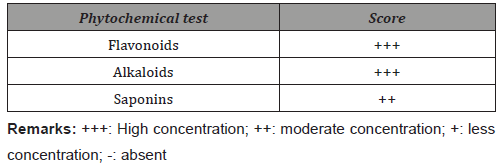
Qualitative analysis of I. palembanica extract showed high amounts of flavonoids, a polyphenol group, in EtOAc extract (Table 2).
Table 2: Qualitative analysis of phytochemicals of Intsia palembanica extract.
Antioxidant activities
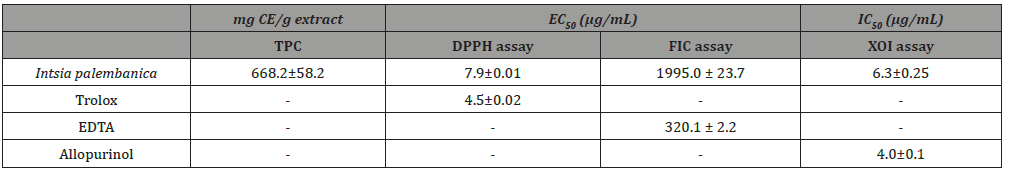
Amounts of phenolic of Intsia palembanica extract was found to be 668.2±58.2 μg catechin equivalents/mg dry weight. When this amount is compared to those found in other studies in the literature, polyphenols seemed to have an important role in stabilizing lipid oxidation and is associated with the antioxidant activity Yen et al. [32]. About 0.7 mg of catechin equivalents of phenol were present in 1 mg of the I. palembanica ethyl acetate extract which may contribute directly to the antioxidative action shown by the extract (Duh et al., 1999). It has been suggested that up to 1 g of polyphenols consumed daily from diet rich in fruits and vegetables have the ability to inhibit mutagenesis and carcinogenesis in humans [33]. The findings indicated that EtOAc was the best solvent to be used for extracting phenolic components from plants. Similar findings were reported by Sim, et al. (2010), Al-Juhaimi, et al. (2013) and Kusirisin, et al. (2009). The reduction capability of DPPH• was determined by the decrease in its absorbance at 517 nm induced by antioxidants. The decrease in absorbance of DPPH⋅ caused by antioxidants is due to the reaction between antioxidant molecules and radical progresses, which results in the scavenging of the radical by hydrogen donation. It is visually noticeable as a discolouration from purple to yellow. Therefore, DPPH• is usually used as a substrate to evaluate antioxidative activity of antioxidants. Trolox was used as standard. The scavenging effect of I. palembanica extract and standards on the DPPH• decreased in the order of Trolox and I. palembanica extract with the EC50 of 4.5±0.02 μg/mL and 7.9±0.01 μg/mL, respectively. The effects of I. palembanica extract reached a plateau at 59.1% as compared to Trolox which reached the maximum at 74.9%. These results indicated that I. palembanica extract has a noticeable effect on scavenging free radical and the free radical scavenging activity also increased with increasing concentration. This effect may be due to the polyphenolic content in the Intsia palembanica extract which include the piceatannol, resveratrol, myricetin and robinetin [9] and the new isolated compounds, robinetinidol, dihydrorobinetin and the two isomers of ampelopsin [(2R, 3S)- and (2R, 3R)-ampelopsin] (Figure 1).
EDTA was used as a standard reference for measuring the iron chelating activity. EDTA is a potent chelating agent of metals. When compared to the test compounds, EDTA showed the best chelating activity because the effect can be seen even at low concentrations. The effective concentration that produced 50% effect value (EC50) of EDTA was 0.032± 0.002 mg/mL (Table 3). However, the EC50 value of of I. palembanica was 19.95±2.37 mg/mL (Table 3). EDTA was 600 times more potent than I. palembanica in chelating iron but, has more potential than trolox and ascorbic acid. I. palembanica exhibited the high xanthine oxidase inhibitory activity with IC50 value of 6.3±2.5 μg/ml (Table 3). Allopurinol, drug used for the treatment of gout, was used as the standard reference. The IC50 value was 4.0±1.0 μg/ml (Table 3) and this IC50 value of allopurinol was comparable to a study done by Umamaheswari et al. (2007), 6.75 μg/ml. This suggests that the I. palembanica inhibitory effect was as potent as allopurinol. Xanthine is a substrate of xanthine oxidase. Xanthine oxidase plays an important role in converting xanthine to uric acid and superoxide anion radical (Figure 2). By adding test compounds in the reaction mixture, the enzyme can be inhibited and consequently lead to a decrease in the production of uric acid and superoxide anion radicals. The higher the concentrations of the tested compounds, the more xanthine oxidase enzymes were inhibited. Nonetheless, the inhibitory effect was saturable at high concentration of the test compounds (Figure 2) (Table 3).
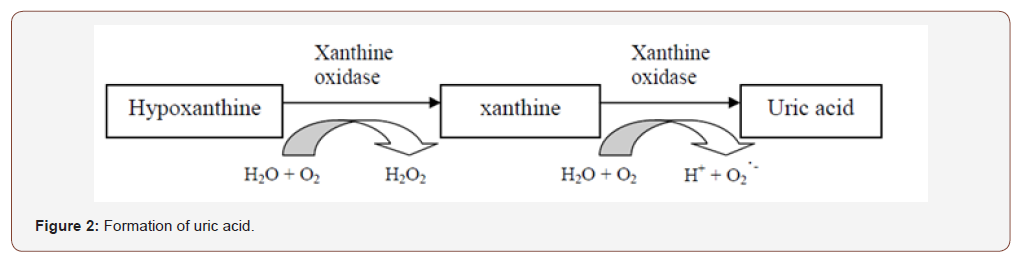
Table 3: Antioxidant activities of Intsia palembanica extract. Values are mean ± SD (n=3).
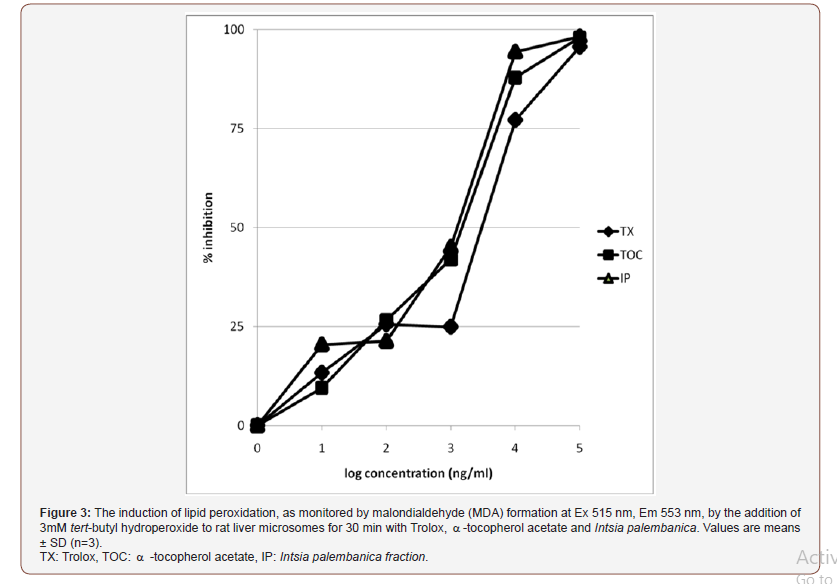
Newly developed xanthine oxidase inhibitors have been highly searched to replace the currently used allopurinol which is used for gout treatment despite its severe adverse effects. Allopurinol is a xanthine oxidase inhibitor applicable exclusively to treat chronic gout and conditions related to hyperuricemia Tan et al., [34]. Many studies have been conducted on plant extracts for a higher therapeutic activity and fewer side effects. It has been reported that crude extracts causing more than 50% xanthine oxidase inhibition at a test concentration of 100 μg/ml are justified for further investigation Sweeney et al. From the experiment, I. palembanica extract exhibited high inhibition activity with an IC50 of 6.3±2.5 μg/ ml, 10 times more potent than the one proposed by Sweeney et al., Tan et al. suggested that most of the natural xanthine oxidase inhibitors present in the active extracts may be polyphenols. I. palembanica extract has been found to contain polyphenol substances [9] and the bark and leaves of I. palembanica were traditionally used in the treatment of rheumatoid, diarrhoea and urinary problems (Soerionegara & Lemmens, 1993; Soon-Leong et al. 2002). Our study also has shown the presence of six polyphenols. This further support the result obtained from the XOI assay as it is traditionally used to treat inflammation. I. palembanica extract had potent inhibitory effect on the hydroperoxide-initiated lipid peroxidation in liver microsomes as compared to α-tocopherol acetate or trolox (Figure 3). The IC50 values of I. palembanica, α-tocopherol acetate and trolox were 1.26, 1.58 and 3.98 μg/mL, respectively. I. palembanica extract showed a maximum inhibition at 98.2%. Trolox and α-tocopherol acetate reached a plateau at 95.6% and 97.2%, respectively. TBARS production of microsomal fractions depended on the amount of tissue, the time of incubation, the concentration of tert-butylhydroperoxide and the presence of antioxidants, if any. TBARS is a good index of lipid peroxidation in rat and rabbit microsomes when compared with the production of hydroperoxides (Tien & Aust 1986) (Figure 3).
This study has demonstrated that I. palembanica extract possessed antioxidant and free-radical scavenging activity. However, it is said that an antioxidant via chelation of Fe2+ is a more significant mechanism in preventing lipid peroxidation than direct free radical scavenging [35]. Certainly, the ethyl cetate extract had the ability to inhibit lipid peroxidation and xanthine oxidase, chelate Fe2+ and/or scavenge DPPH•. Then again, the extract showed different behaviour in different assays, probably due to the different mechanisms involved. It is inappropriate to use only one method to assess antioxidant capability of a sample because the antioxidant activity measured by an individual assay exhibits only the chemical reactivity under specific conditions applied in that particular assay [36,37]. Oxidative stress in cells can be reduced by antioxidants and this notion is useful in treating many human diseases, including cancer, cardiovascular diseases and inflammatory diseases (Krishnaiah et al, 2011). Dietary polyphenols play a role in the prevention of degenerative diseases, particularly cardiovascular diseases and cancers and has been considerably developed for the past 15 years Krishnaiah et al. [38]. Although the antioxidant properties has been extensively studied, the mechanism of actions of the polyphenols are past the modulation of oxidative stress [5]. Several studies have investigated the relationship between plant products and their antioxidant activities. Positive correlations can be seen in a lot of studies between the phenolic content and the antioxidant activities exhibited. High antioxidant capacities may be contributed by unidentified compounds and their synergistic interactions with other polyphenols present in the plant products leading to health improvement and disease prevention [39] Valentova et al. The aromatic rings in the polyphenol structure with one or more hydroxyl groups and other functional groups may be the contributing factor (Rice-Evans et al. 1996). These structures are able to scavenge ROS (Halliwell, 1999; 2000), inhibit lipid peroxidation (Jovanovic et al., 1994; Fraga et al., 1987) and chelate metal ions (Terao et al., 1994; Halliwell, 1995) depending on the number and the location of the hydroxyl groups. Polyphenols inhibit oxidation processes via: (i) chelation of metal ions by the O-hydroxy phenolic structure; (ii) scavenging of alkoxyl lipid radical and peroxyl radical by terminating chain reaction, or (iii) regeneration of α-tocopherol through reduction of α-tocopheroxyl radical (Rice-Evans, 1995).
The need to improve human health and quality of life gave rise to studies on the biological activities of polyphenols, in vivo and in vitro. Polyphenols present in red wine and tea have been shown to have potent antioxidant characteristics El Attar and Virji, [40]. Tea is a rich source of polyphenols, more potent than other vegetables and fruits that could inhibit LDL oxidation caused by ROS which can lead to atherogenesis Ishikawa et al., Scalbert et al., [41,42]. This may be due to the catechol moiety existed in the polyphenol compound in tea (o-hydroxy or o-trihydroxypenyl rings) Ishikawa et al., Javonovic et al., [43]. Medicinal plants have played an important role in the culture of healing human and livestock diseases, traditionally. From these claims of almost 80% from the world’s population in developing countries, many investigations have been carried out to prove scientifically the efficacies of these plants which have consistently been an important source of therapeutic agents. Hence, at present, about 25–30% of all drugs available as source of therapeutics are derived from plants, microbes and animals or are natural product derivatives [15,44,45]. Over 490 medicinal plant species from Fabaceae (second largest family of medicinal plants) nhave been used as traditional medicines [46]. Some ethnomedicinal plants from the family Fabaceae have been identified for the use of treating diabetes, diarrhea, dysentery, fever, arthritis, goitre, skin disease and many others in India. The root paste of Flemingia macrophylla was reported to be applied on affected areas for people suffering arthritis and the stem bark decoction for treating fever (Tirkey, 2006). The same plant was also claimed to have anti-inflammatory and antidiabetic activities by the Taiwanese and have demonstrated potent anti-hyperglycemic activity [47]. The flavonoids from Flemingia macrophylla also had neuroprotective activity against Alzheimer’s disease [48,49]. The tubers of Hydnophytum formicarum Jack from the Rubiaceae family has been used traditionally to treat hepatitis, rheumatism and diarrhea in South East Asia [50-52]. The ethyl acetate extract of this tuber demonstrated high antimicrobial and antioxidant activities due to the presence of phenolic compounds such as isoliquiritigenin, protocatechualdehyde, butin and butein [53].
Therefore, it is strongly suggested that the polyphenols in the ethyl acetate fraction of I. palembanica are protecting cells against any damage induced by oxidizing agents such as H2O2 and metal ions (ferrous ions). Damage done by ROS and transition metal ions could influence the plasma thiol level, antioxidant enzyme activity, protein carbonyl content, and fluidity of erythrocyte membrane [54,55]. The ethyl acetate extract of I. palembanica was able to scavenge DPPH• and t-BuOO•. Number of hydroxyl groups and the glycosylation process of the polyphenol structure and the ability to chelate Fe2+ contributed to the inhibition [55], Halliwell and Gutteridge, Rice-Evans et al. The ability of I. palembanica extract to inhibit TBARS generation seems to be related to their ability of scavenging lipid radicals in rat liver microsomes. Dietary plant polyphenols have been shown to modulate lipid and carbohydrate metabolism, alleviate insulin resistance and hyperglycemia. These polyphenols can also improve oxidative stress and stress-sensitive signallig pathways and inflammatory processes [56]. These findings proved that the presence of polyphenols in I. palembanica have potent antioxidant activities and react through the radical scavenging mechanism more than the chelation of Fe2+. This is shown by the identification of six polyphenol compounds from the ethyl acetate extract such robinetinidol, dihydrorobinetin, two isomers of ampelopsin [(2R, 3S)- and (2R, 3R)-ampelopsin], robinetin and myricetin. Furthermore, a study done by [57] have confirmed a strong radical scavenger in ABTS+. assays: 34.11 ± 0.02 mM Trolox equivalents per g of dry extract in the extract of merbau wood [57]. Hence I. palembanica may be a powerful free radical inhibitor or scavenger, as well as a primary antioxidant that reacts with free radicals, which may limit free radical damage occurring in the human body [58-63].
Conclusion
The results presented here with Intsia palembanica extract suggested that the potentiality to be successfully used in human health in the prevention of diseases involving free radical and oxidative damage are quite promising. These findings should be taken into consideration to develop protective antioxidants that may have significant applications in fighting against human diseases caused by free radicals.
To read more about this article...Open access Journal of Pharmacy & Pharmacology Research
Please follow the URL to access more information about this article
To know more about our Journals...Iris Publishers





No comments:
Post a Comment