Authored by Abd Alkareem Omar Maghmomeh*,
Abstract
The incidence of acute kidney injury (AKI) has been increasing over time. AKI increase the risk of progression of chronic kidney disease. Acute and chronic renal failures are global public health issues with different features to take into account in different parts of the world. Curcumin has renal protective properties against kidney damage. The exact mechanism of such protection is not clear. Therefore, this study was conducted to examine the molecular levels of the protective effect of curcumin on cisplatin induced nephrotoxicity in rats. We investigated the effects of curcumin in AKI which was induced in male Sprague Dawley rats using cisplatin (4.5 mg/ kg in 2 consecutive days). Curcumin was administered by oral tubes 200mg/kg daily for 21 days. Serum creatinine, creatinine clearance, urea nitrogen, oxidative stress markers [glutathione (GSH), lipid peroxide (Malondialdehyde)] assessments of these parameters were carried out to indicate the effect of curcumin in AKI. Serum analysis revealed an alteration in parameters of kidney. A decrease in the antioxidant activity of kidney was recorded in cisplatin group while curcumin administration restored it. The results clarified the strong protective effect of curcumin on cisplatin induced nephrotoxicity in rats at the molecular levels.
Keywords: Acute kidney injury; Oxidative stress; Curcumin
Introduction
Acute kidney injury might occur with the use of several drugs, such as non-steroidal anti-inflammatory drugs, antibiotics, antineoplastic drugs and angiotensin-converting-enzyme inhibitors [1]. Severe, long, and repeated episodes of acute kidney injury increase the risk of progression of chronic kidney disease. Acute and chronic renal failure are global public health issues with different features to take into account in different parts of the world, renal complications, which involve most organ systems, can be treated and prevented, by using different therapeutic strategies [2]. Cisplatin is one of the most widely used and most effective cytotoxic agents in the treatment of a variety of malignant tumors, including lung, colorectal, ovarian, breast, head/neck, bladder, and testicular cancers in both children and adults [3]. On the other hand, cisplatin drugs have two main limitations: their severe side effects and the ability of cancers to develop drug resistance. Common side effects of cisplatin drugs include nausea, vomiting, and diarrhea, myelosuppression, neuropathy, ototoxicity, hepatotoxicity and nephrotoxicity [4]. Patients develop acute kidney injury [5] which may progress to chronic kidney injury [6]. Renal involvement is common after cisplatin injection (up to 30-50% of the cases), and it most often occurs in the second week of treatment [7]. Renal damage has a wide spectrum of sign symptoms e.g. hematuria, proteinuria, glucosuria, hypomagnesemia and most notably acute kidney injury [8]. Cisplatin-induced nephrotoxicity may range from mild and reversible structural alterations in tubular epithelial cells inducing a variable range of renal dysfunction (acute nephrotoxicity), to potentially irreversible renal failure leading to chronic and progressive renal insufficiency (chronic nephrotoxicity) [9]. Curcumin is a naturally occurring compound derived from the rhizomes of Curcuma longa. It is a member of the ginger family Zingiberaceae, found in the rhizome of the herb Curcuma longa, which is commonly known as turmeric [10]. Turmeric is widely used in therapeutic preparations [11]. Curcumin has been found to possess several properties including antioxidant [12], antimicrobial [13], antiviral [14], anti-inflammatory [15], anti-carcinogenic [16] and anti-diabetic [17]. Curcumin showed hepatoprotective activity against liver damage in animals induced by carbon tetrachloride [18]. In the present study, we tested curcumin in an in vivo model of cisplatin-induced kidney nephrotoxicity to assess its potential renoprotective effects.
Materials and Methods
Chemicals and kits
Curcumin was purchased from Sigma-aldrich (St. Louis, MO, USA). Creatinine, urea, malondialdehyde (MDA), reduced glutathione (GSH) and phosphate buffer saline (PBS) were purchased from Bio-diagnostic Co (Dokki, Giza, Egypt). Thiopental sodium was supplied in the form of (Anapental 500 mg/vial), purchased from Sigma Tec Co., Egypt.
Animals and experimental protocols
This study comprised of 30 male Sprague Dawely rats 3 months old, whiting (225±25 gm), were utilized in the present study. Rats were housed in stainless steel rodent cages at room temperature (25 ± 2ºC) and with 12 hours dark/light cycle and were provided with standard rat food and water. This study was carried out in strict accordance with the guidelines and authorization for the use of laboratory animals. The protocol was approved by the committee on the ethics of animal experiments of Faculty of Pharmacy, Mansoura University, for Animal Use. The rats were divided into 3 groups (n=10) a) control group: rats were maintained on normal pellet diet. b) cisplatin group: rats were treated with was cisplatin (4.5 mg/ kg .day/i.p) for 2 consecutive days [19] c) cisplatin + curcumin: (4.5 mg/ kg .day) in 2 consecutive days [19], after the one day of cisplatin injection [20], rats were orally administered curcumin was suspended in (PBS) (200mg/kg/day/) for 21 days. The dose and duration were selected according to a previous study [21]. At the end of study, the rats were placed in a metabolic cage for 24 hours to collect 24-hour urine, and samples were taken for estimating urine creatinine [22]. After 12 hours of fasting rats were sacrificed after anesthetization using thiopental sodium (40 mg/kg, IP of 2.5 % thiopental). Blood samples were withdrawn from thiopental-anesthetized animals via retro-orbital puncture after a fast of 12 hours [23]. Serum was extracted after blood centrifugation for 10 min at 4000 × g. Kidney tissues were removed was homogenized in 5 ml ice-cold PBS (0.02 M, pH 7.4) (10% w/v), centrifuged at 3000 rpm for 20 min at 4 ˚C and kept at -80˚C until further analysis.
Determination of biochemical parameters
Serum creatinine, urea, kidney malondialdehyde (MDA) and reduced glutathione (GSH) were assayed using calorimetric kits according to manufacturer’s instructions.
Statistical analysis
Data were expressed as a mean ± standard deviation (M ± SD) in each group. Statistical evaluations of the results were carried out by means of one-way analysis of variance (ANOVA), followed by Turkey multiple comparison tests. Statistical tests were performed using the Statistical Package for the Social Sciences (SPSS) version 13 (Chicago, IL, USA). Statistical significance was taken at P < 0.05 and P < 0.01. Graphing was carried out using GraphPad Prism software (Graphpad Software Inc., San Diego, USA).
Results
Effect of curcumin on kidney function
As shown in Figure 1-3, serum creatinine and urea levels were significantly increased, however, creatinine clearance was significantly reduced in Cis group when compared to the control group (p< 0.01). Curcumin treatments were significantly reduced serum creatinine and urea levels and significantly increased creatinine clearance level compared to Cis group (p< 0.05) (Figure 1-3).
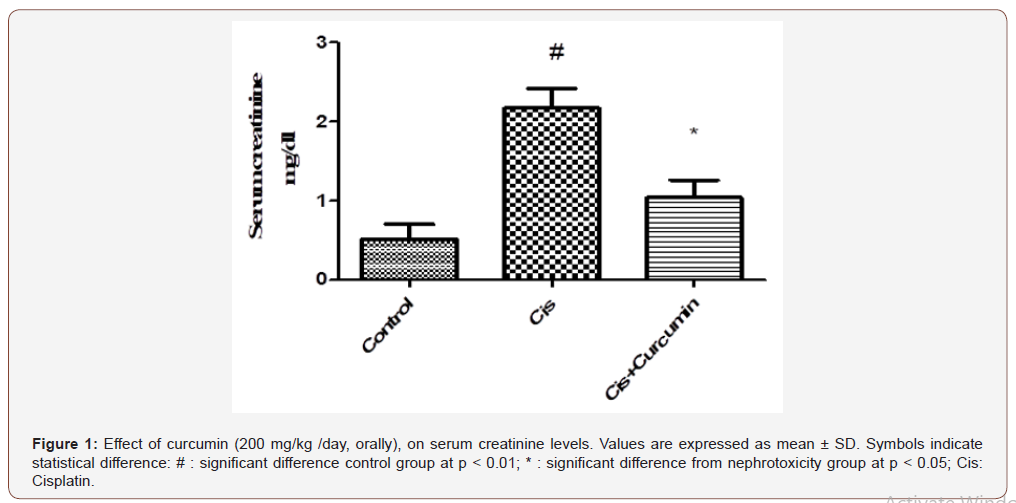
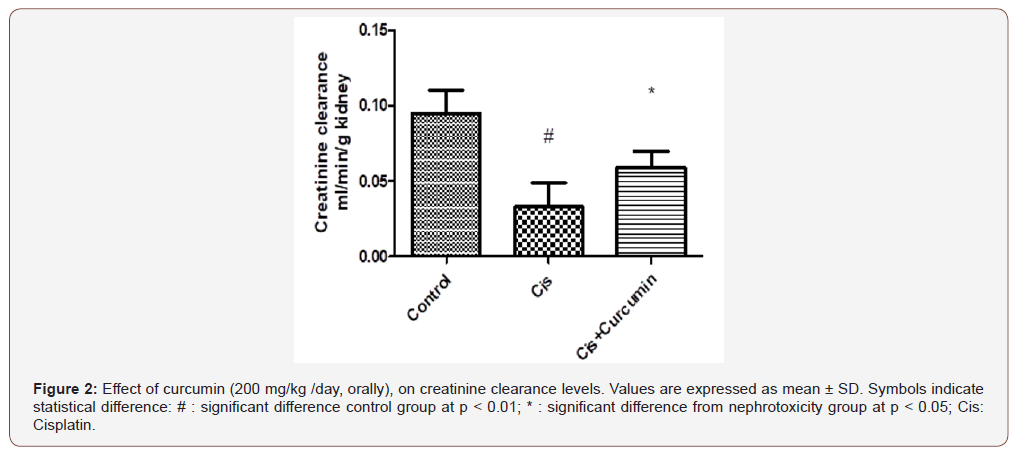

Effect of curcumin on oxidative stress
As shown in Figure 4-5, kidney MDA was significantly increased however; GSH was significantly reduced in Cis group when compared to the control group (p< 0.01). Curcumin treatments were significantly reduced MDA and were significantly increased GSH compared to Cis group (p< 0.05) (Figure 4-5).
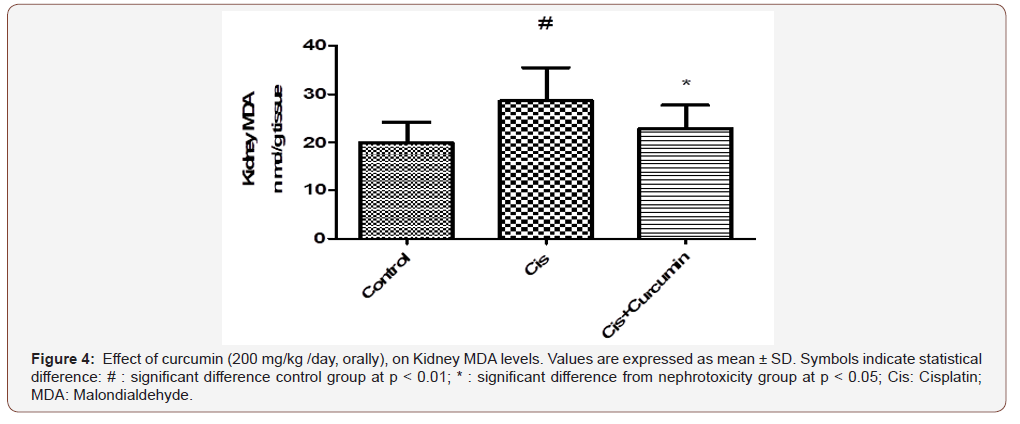
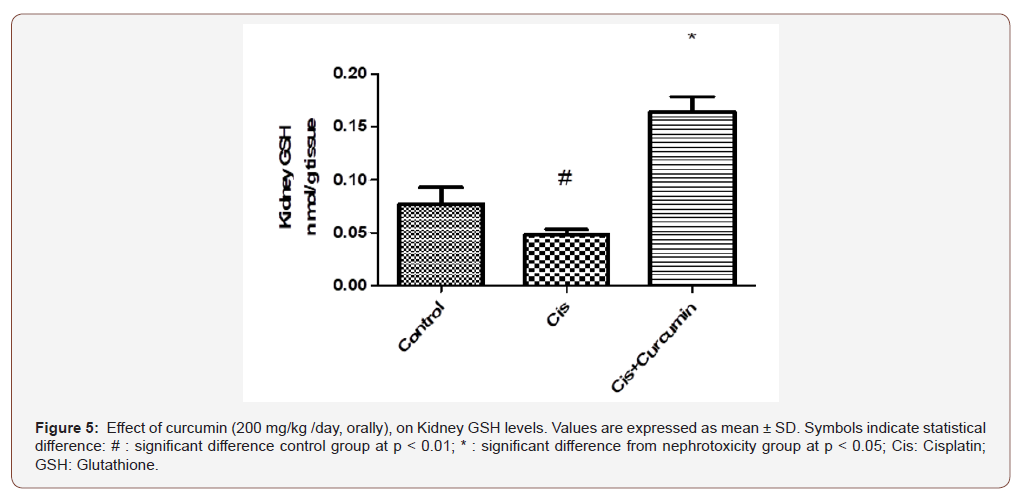
Discussion
Cisplatin is used to manage several human malignancies. However, nephrotoxicity is a serious side effect that limits its use as a chemotherapeutic agent. This may require reducing the dose or resorting to alternative treatment strategies [24]. Cisplatin induces glomerular and tubular dysfunctions [25] and injury of renal vasculature and structures [26]. Cisplatin causes tubular epithelial cell toxicity, vasoconstriction in the renal microvasculatureand inflammatory effects [27]. Cisplatin was also found to decrease glomerular filteration rate possibly due to its nephrotoxic effect on the S3 segment of the proximal tubule [28]. Patients develop acute kidney injury [5] which may progress to chronic kidney injury [6, 29]. In our study, serum and urine levels of creatinine and urea nitrogen were altered after cisplatin treatment indicating kidney injury. The evaluation of renal function and glomerular damage involved the examination of serum creatinine levels and reduction in creatinine clearance in accordance with previous reports [30]. Creatinine is a waste product of metabolism excreted by the kidneys without reabsorption. Hence, it is used as an index of renal function and to monitor renal dysfunction or damage [31]. Our study showed that treatment with cisplatin for (4.5 mg/ kg. day) rats’ group [19] two days was sufficient to induce nephrotoxicity in rats [20] as indicated by elevated serum creatinine and reduced creatinine clearance. Curcumin was found to mitigate cisplatin-induced kidney injury in pretreated rats as evidenced by reduction in serum creatinine and blood urea nitrogen. This is in agreement with previous studies [32]. Curcumin treatment ameliorated the former parameters. Creatinine and urea levels decreased significantly whereas creatinine clearance levels increased significantly during the treatment when compared with the nephrotoxicity groups. Oxidative stress plays a key role in cisplatin-induced renal dysfunction. Cisplatin enhances the production of superoxide, peroxynitrite, hydrogen peroxide and hydroxyl radicals via mobilization of iron from renal cortical mitochondria [22]. The enhanced expression of reactive oxygen species (ROS) by cisplatin is directly related to tubulointerstetial fibrosis [33]. Cisplatin is activated when it enters the cell through binding to the N7 reactive center on purine rings, resulting in DNA damage in cancer cells, blocking cell division and contributing to apoptotic cell death [34]. Cisplatin also induces ROS-mediated cell death [35]. ROS production increases during cisplatin treatment of cultured renal tubular cells, kidney slices, and in vivo in whole animals [36].
Conclusion
As a result of increased ROS production by cisplatin, antioxidant enzymes such as GSH are depleted [37]. Cisplatin also triggers MDA production in renal tissue [30]. MDA is a reactive aldehyde and forms covalent protein adducts which are referred to as advanced lipoxidation end products, very similar to advanced glycation end products [38, 39] It is the product of lipid peroxidation and reflects the degree of oxidation in the body. GSH is a tripeptide abundant in cells and is responsible for modulation of cell proliferation, antioxidant defense and detoxification of drugs and toxins. GSH also prevents damage to important cellular components caused by reactive oxygen species such as free radicals, peroxides, lipid peroxides and heavy metals [40]. Cisplatin can produce ROS through different mechanisms. Cisplatin can rapidly react with thiolcontaining molecules, primarily glutathione [41]. Once glutathione and other antioxidants are consumed, a shift in the cellular redox status occurs. ROS then accumulates within the cell and causing a state of oxidative stress. [42]. ROS may also disrupt the respiratory chain and cause mitochondrial dysfunction. The cytochrome P450 system has also been reported to be responsible for generating ROS as demonstrated in in vitro and in vivo models [43, 44]. In this study, the antioxidant status was assessed by measuring GSH and MDA levels. Kidney MDA was significantly increased in cisplatin group compared to control group. However, GSH was significantly reduced in the cisplatin group when compared to the control group. Curcumin treatment significantly reduced MDA and were significantly increased GSH compared to cisplatin group (p< 0.05).
Curcumin is known for its high oxygen-radical scavenging and quenching power [12]. It is a scavenger of free oxygen radicals and stimulates the activity of additional antioxidant molecules such as superoxide dismutase, catalase, and glutathione peroxidase [45]. It is a bifunctional antioxidant [46] because of its ability to react directly with reactive species and to induce an up regulation of various cytoprotective and antioxidant proteins [47]. Curcumin can react with ROS through its phenolic and methoxy groups and it is thought to be one of the mechanisms through which it can protect the renal epithelial from ROS activity [48]. Curcumin can indirectly induce the expression of cytoprotective proteins such as superoxide dismutase (SOD), catalase (CAT) [49], GSH [50], nicotinamide adenine dinucleotide phosphate (NADPH), quinone oxidoreductase 1 (NQO1) [51]. Furthermore, it has been reported that curcumin can increase the synthesis and concentration of GSH [52]. In summary, this study demonstrated that curcumin can protect against nephrotoxic effects of cisplatin in rats. The protective effect of curcumin occurred through the up regulation of antioxidants and suppression of oxidative stress markers. Curcumin is a promising therapy for management of kidney nephrotoxicity. Further in vitro studies are needed to outline the signaling pathways involved in curcumin actions during nephrotoxicity.
To read more about this article...Open access Journal of Pharmacy & Pharmacology Research
Please follow the URL to access more information about this article
To know more about our Journals...Iris Publishers





No comments:
Post a Comment