Authored by Hua Yang*,
Abstract
Herb extract is a type of well-known natural antimicrobial from plants. Food Drug Administration recognized that most herb extracts as Generally Recognized as Safe for human consumption. The objective of this study is to conduct three experiments and to evaluate the inhibitory and bactericidal effects of nine herb extracts against five representative strains of Listeria monocytogenes in vitro. In the experiment 1, each of herb extracts 2, 4, 5, 8 exhibited inhibitory effects against five strains of L. monocytogenes individually at 37 °C in Mueller-Hinton broth (MHB). The MIC values of those four herb extracts ranged between 5 - 50 mg/mL. In experiment 2, herb extract 4, which showed the lowest MIC value (5 mg/mL), reduced populations of L. monocytogenes in a range of 0.38 - 0.91 log CFU/mL after 30 min treatment at 37oC in MHB, indicating that herb extract 4 may not expected to be used as an antimicrobial agent for the purpose of reducing L. monocytogenes within a short period of time. In experiment 3, at concentrations of 1.56 and 0.78 mg/mL, herb extracts 2, 4, 5, 8 inhibited the growth of a five-strain L. monocytogenes cocktail individually at the abused refrigerator temperature of 12 oC, except herb extract 8 at the concentration of 0.78 mg/mL. At a concentration of 3.13 mg/mL, those four herb extracts reduce cell populations in a range of 2.2 to 1.6 mg/mL at 11 days. Herb extracts 2, 4, 5 and 8 could be potentially developed into food preservatives for controlling foodborne L. monocytogenes.
Keywords: Antimicrobial effect; Herb extracts; Listeria monocytogenes
Abbreviations: Herb extract (HE); Colony forming unit (CFU); Minimum inhibitory concentration (MIC); Mueller-Hinton broth (MHB); Ready-to-eat (RTE); Transfers in tryptic soy broth (TSB); Tryptic soy agar (TSA); Buffered peptone water (BPW); Phosphate-buffered saline (PBS)
Introduction
Listeria monocytogenes is a gram-positive foodborne pathogen that is widely distributed during food preparation, storage, and distribution. A variety of ready-to-eat (RTE) foods such as milk, cheeses, ice cream, raw meat, fresh vegetable and fruits may be contaminated with Listeria monocytogenes [1,2]. Consumption of foods contaminated with L. monocytogenes is linked to an increased risk of listeriosis. To control L. monocytogenes in food products, meat industry uses chemical preservatives such as sodium acetate, sodium lactate and various nitrites. However, it is acknowledged that uses of chemical antimicrobials have increased the consumer concerns and created a demand for “natural” and “minimally processed” food. As a result, there has been a great interest in natural antimicrobial agents.
Plant-derived extracts have been used since ancient times, especially in China [3] and India [4,5]. In addition to the uses as flavoring material, plant extracts and essential oils represent a natural alternative in the nutritional, pharmaceutical, and agricultural fields. Due to their antimicrobial properties, plant extracts have been suggested to be used as antioxidant and preservatives in food products, to incorporate into food packaging materials, plant and crop protectants against insect pests, and medicinal products for human and livestock [6]. In recent times, plant extracts have gained great interests especially in food industry. Most plant extracts are classified as generally recognized as safe by U.S Food and Drug Administration, and are easily degradable in human body [7,8]. Previous studies have been proven that many spices and plant essential oils exhibited inhibition and/or bactericidal effects against L. monocytogenes in food products. For example, cinnamon essential oil and oregano reduced the growth rate of L. monocytogenes by 10% and 19% respectively in ham at 4 °C [9]. Thyme and clove essential oils reduced populations of L. monocytogenes in zero-fat beef hotdogs by 1.3 log CFU/g and 1.0 log CFU/g respectively with 5 min treatment at room temperature (21 °C) [10]. The objective of this study is to evaluate potential inhibitory and bactericidal effects of nine herb extracts (HEs) against foodborne pathogenic L. monocytogenes in vitro in order to select natural antimicrobial agents for the control of foodborne L. monocytogenes in food products.
Introduction
Experimental design
HEs can be used to inhibit the growth of foodborne pathogen and/or reduce pathogen populations. In this study, we conducted three experiments to evaluate their potential uses as antimicrobial agents in food products. In experiment 1 (Exp. 1), a minimum inhibitory concentration (MIC) study was conducted to compare inhibitory effects of each of nine HEs against each of five L. monocytogenes strains individually in Mueller-Hinton broth (MHB). In experiment 2 (Exp. 2), the HE with the lowest MIC was used to determine its reductions of each of five L. monocytogenes strains individually at 37 °C for 30 min in MHB. In experiment 3 (Exp. 3), those HEs which could inhibit the L. monocytogenes growth in Exp. 1 were evaluated for their inhibitory effects against a five-strain L. monocytogenes cocktail in MHB up to 11 days at 12 °C.
Bacterial strains and growth conditions
Table 1: Bacterial strains used in the study [15].
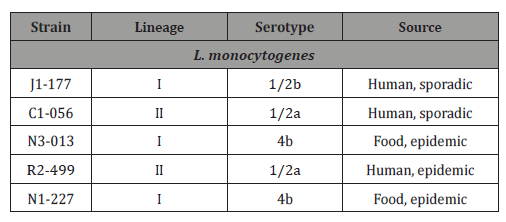
Five strains of L. monocytogenes which isolated from epidemics were used in this study and are listed in Table 1. According to [11], these five L. monocytogenes strains were selected from a total of 46 strains which represented a genetic diversity of ribotypes, pulsedfield gel electrophoresis types, serotypes, and lineages. In addition, these five strains are believed to cover the genetic diversity of human disease- associated L. monocytogenes and to provide a valuable tool for evaluating the effectiveness of antimicrobials to inactivate or inhibit L. monocytogenes. Therefore, we used these five genetically distinct strains of L. monocytogenes to evaluate inhibitory and bactericidal efficacies of nine HEs. All strains were activated from 20% glycerol frozen stocks (-80 °C) by two transfers in tryptic soy broth (TSB) (Difco, Spark, MD) at 37 °C for 24 h and were subsequently subculture on tryptic soy agar (TSA) (Difco, Spark, MD) at 37 °C for 24h. Each activated strain was kept on TSA plates at 4 °C.
Herb extracts preparation
A total of nine types of herbs were obtained in the form of powder. Each of nine herbs was extracted with sterile deionized water followed by the procedure of [12] with modification. The HEs were prepared before the day of experiment. Each of the HEs was made by combining 10g of each herb powder with 90 mL of sterile deionized water, incubating in a water bath at 45 °C for 30 minutes, and then boiling for 15 minutes. Each of the nine HEs was then cooled to room temperature and was centrifuged at 6000 x g for 15 minutes at room temperature (Thermo Scientific Sorvall Legend X1R Centrifuge, Am Kalkberg, Germany). The supernatant of each HE was transferred into a 50 mL polypropylene tube and stored at 4 °C until use next day.
Exp. 1: Determining MICs of the HEs
Each strain of the L. monocytogenes listed in Table 1 was inoculated in TSB individually and was incubated at 37 °C for 24h. After the incubation, each strain was serially diluted in MHB (Difco, Spark, MD) to approximately 106 CFU/mL. Nine HEs were diluted with the sterile deionized water to six concentration levels: 100, 60, 30, 15, 10, 5 mg/mL. Five mL of each diluted strain was mixed with 5 mL of each diluted HEs in glass sterile test tubes, to make the final concentrations to be 50, 30, 15, 7.5, 5, 2.5 mg/mL for each HEs and approximately 5 x 105 CFU/mL for each strain. Negative control samples were prepared by combining 5mL of each of nine diluted HEs with 5 mL of MHB separately to make the same final herb concentrations for each HE listed above but without inoculum. Positive control samples were prepared by combining 5mL of each diluted strain with 5mL of MHB separately to make same final bacteria concentrations for each diluted strain listed above but without any HE. All tubes were subsequently incubated at 37 °C for 24h. After 24h incubation, all treatment and control samples were visually examined. The lowest herb concentration at which each treatment sample did not show turbidity were designated as the MIC. All tests were performed in two independent replication trails with three samples on each trail (n=6).
Exp. 2: Reduction of L. monocytogenes cells treated with the HE 4
The HE 4 exhibited inhibitory effect against L. monocytogenes with the lowest MIC in Exp. 1. In this experiment, the HE 4 was determined for its reduction of L. monocytogenes cells. After the 24h incubation, each strain was serially diluted in MHB to approximate concentration of 106 CFU/mL. The HE 4 was diluted in sterile deionized water to the concentration of 50 mg/mL. Two mL of each of diluted L. monocytogenes strains was combined with 2mL of the diluted the HE 4 separately, to make a final concentration of 25 mg/mL of the HE and approximate 5 x 105 CFU/mL of each strain. The positive control samples were prepared by combining 2mL of sterile deionized water and 2mL of each of the five diluted strains separately, to make the same concentrations of each strain as the treatment samples but without HE 4. All treatment and control samples were incubated for 30 min at 37 °C. Our preliminary data showed that the HE 4 exhibited the best reductions against each of five L. monocytogenes strains at 37 °C (data not shown). In a previous published study, thyme and clover have been reported to reduce populations of L. monocytogenes after 5 min treatment in peptone water at room temperature (21 °C) [10]. In our study, each of five strains were treated 30 min with HE 4, which was six times longer than [10]. After 30 min treatment, all treatment samples were immediately diluted with sterile deionized water to a concentration of 0.25 mg/mL for the HE 4, in order to terminate its further antimicrobial activity. Our preliminary study has shown at the concentration of 0.25 mg/mL, the HE 4 could not inhibit the growth of each of five L. monocytogenes strains (data now shown). Each of treatment and positive control samples was subsequently serially diluted in 0.1% buffered peptone water (BPW) and each diluted sample were then plated onto tryptic soy agar (TSA) with two duplications. The TSA plates were then incubated for 48 h at 37oC to enumerate the numbers of surviving L. monocytogenes cells. All tests were performed in two independent replication trails with two samples on each trail (n=4).
Exp. 3: Antimicrobial effects of HEs against L. monocytogenes cocktail at abused refrigerated temperature
In Exp. 1, HEs 2, 4, 5 and 8 which inhibited L. monocytogenes growth at or below 50 mg/mL concentrations. In this experiment, those four HEs were evaluated for their inhibitory effects at 12 °C, which represents the abused refrigeration temperature. Each of the five L. monocytogenes strains listed in Table 1 was cultured in TSB separately for 24h at 37 °C. A five-strain L. monocytogenes cocktail was prepared prior to the study. A 10-mL volume of each 24h grown culture was pooled and mixed in a 50 mL sterile falcon tube. After centrifugation at 6000 x g for 15 min at 4 °C, the supernatant was removed. The cell pellet was washed once with a 10-mL volume of phosphate-buffered saline (PBS), and subsequently resuspended in 50 mL PBS. The L. monocytogenes cocktail was serially diluted in MHB to an approximate 5 x 102 CFU/mL concentration.
The HEs 2, 4, 5, and 8 were diluted in sterile deionized water to three levels of concentrations, 6.25, 3.13 and 1.56 mg/mL. Concentrations of HEs were determined based on the preliminary data (data not shown). The treatment samples were prepared by combining 2 mL of each of four diluted HEs and 2mL of the diluted L. monocytogenes cocktail separately in glass test tubes, to make the final concentrations of each of the four HEs at three levels, 3.13, 1.56 and 0.78 mg/mL, and approximately 2.5 x 102 CFU/mL of the L. monocytogenes cocktail. The positive control samples were prepared by combining 2 mL of diluted L. monocytogenes cocktail and 2 mL of sterile deionized water separately but without any HE. Surviving cells from control samples were enumerated immediately after inoculation (day 0). All treatment and control samples were incubated for up to 11 days at 12 °C. All samples were serially diluted in 0.1% BPW and subsequently plated onto two duplicate TSA plates daily from day 1 to day 5, and every two days from day 7 to day 11. TSA plates were incubated for 48h at 37 °C to enumerate the numbers of surviving L. monocytogenes cells. Each treatment sample and control sample were performed in three independent replication trails with two samples on each trail (n=6).
Statistical Analysis
Microbiological data were converted to log CFU/mL prior to the statistical analysis. Statistical analyses were conducted using analysis of variance via the glimmix procedure of SAS (SAS Studio Basic Edition 3.8, SAS Institute, Inc., Cary, N.C.). Least square means were calculated and significant differences between means were detected at the P < 0.05 in the Exp. 2 and at P < 0.001 in the Exp. 3.
Results and Discussion
MICs of nine herb extracts
MIC is defined as the lowest concentration of an antimicrobial agent which prevents visible microbial growth under designed conditions [13]. In this study, the visible microbial growth was determined by comparing the turbidity between treatment samples and control samples after 24h incubation at 37 °C. The MIC value for each of the nine HEs against each strain are shown in Table 2. Four HEs 2, 4, 5 and 8 inhibited the growth of the five L. monocytogenes strains at MIC values ranging from 5 to 50 mg/mL. The other five HEs 1, 3, 6, 7, 9 did not exhibited inhibition effects at up to 50 mg/ mL. Based on the MIC values, the inhibitory effects of those four HEs were ranked from the strongest to weakest as follows: HE 4 (5 mg/mL) > HE 5 (15 mg/mL) > HE 2 (15-30 mg/mL) > HE 8 (50 mg/mL).
Table 2: Minimum inhibitory concentration of the nine herb extracts against five L. monocytogenes strains (n=6).
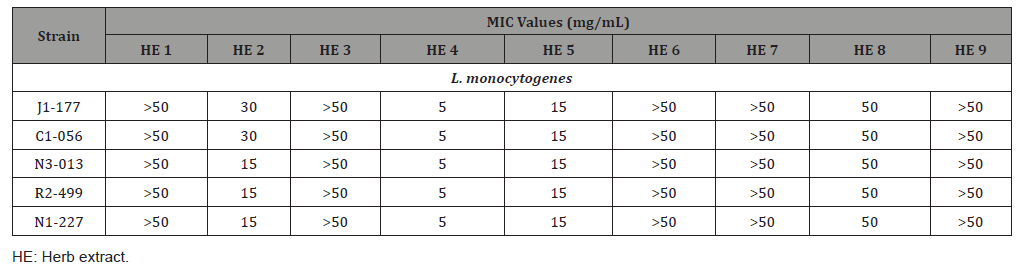
The sensitivity to different natural antimicrobials varies in some Gram-positive and Gram-negative bacteria. For example, studies have shown that Gram-positive L. monocytogenes were more sensitive to some essential oils and HEs than Gram-negative E. coli and Salmonella enterica Enteritidis [14-16]; The Ocimum sanctum extract was found to be equally effective against Gramnegative bacteria (E. coli, S. typhimurium and P. aeruginosa) and Gram-positive bacteria (Staphylococcus aureus) [17]; however, Gram-negative pathogens, V. parahaemolyticus and S. typhimurium, were more sensitive to eugenol than Gram-positive S. aureus [18]. As a result of Exp. 1, four out of nine HEs inhibited the growth of L. monocytogenes. Further studies can be conducted to evaluate and compare the antimicrobial effects of those nine HEs against other foodborne Gram- positive and Gram-negative pathogens.
Reduction of L. monocytogenes cells treated with HE 4
In Exp. 2, the HE 4 was chosen to evaluate its reductions of five L. monocytogenes strains individually at 37 °C for 30 min treatment since HE 4 exhibited the strongest inhibition effect with the lowest MIC (5 mg/mL) in Exp. 1. After 30 min incubation with HE 4 at a concentration of 25 mg/mL, differences (P < 0.05) of surviving cells between treatment samples and control samples were observed for each of five L. monocytogenes strains (Figure 1). Cell reductions of HE 4 against five L. monocytogenes strains were calculated: N1-227 (0.91 log CFU/mL), C1-056 (0.87 log CFU/mL), R2-499 (0.85 log CFU/mL), J1-177 (0.59 log CFU/mL), N3-013 (0.38 log CFU/mL).
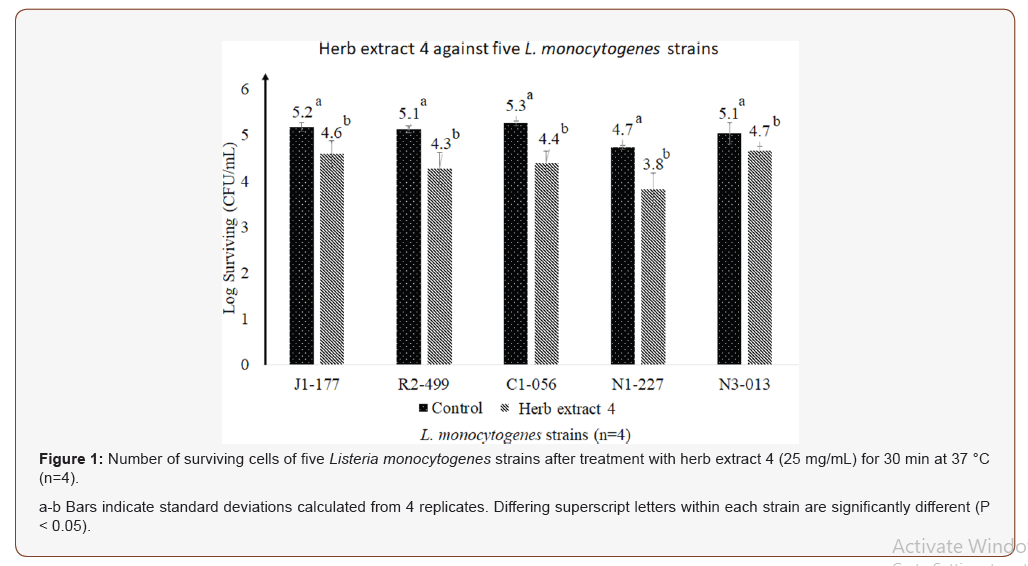
In a previous published study, at the concentrations of 0.5 mL/L, essential oils of thyme and clover have been reported to reduce populations of L. monocytogenes from 7.2 to 1.8 log CFU/mL and from 7.1 to 1.2 log CFU/mL respectively after 5 min treatment in peptone water at room temperature (21 °C) [10]. In addition, another study indicated that essential oil of origanum reduced populations of each of five L. monocytogenes strains in a range of 1-2 log CFU/mL after 30 min treatment in 0.9% saline solution at room temperature [19]. In our study, Although HE 4 reduced less than 1 log CFU/mL for each strain, populations of surviving cells of each strain were significant (P < 0.05) after HE4 treatment compared with control samples. The result indicated that using HE 4 solely against L. monocytogenes might be less effective than essential oils of thyme, clover and organum. However, there has been increased interests to the use natural antimicrobial agents in their combinations for controlling foodborne pathogens. The effects of the combined substances were observed to be greater than the sum of individual effects against L. monocytogenes in combinations of carvacrol/linalool [20] and oregano/rosemary [21]. HE 4 was expected to be used in combination with other compounds to increase antimicrobial effects.
Inhibitory effects and reductions of four herb extracts against L. monocytogenes cocktail at abused refrigerated temperature
Since HE 2, 4, 5 and 8 exhibited inhibitory effects against L. monocytogenes at 37 °C in Exp. 1, we expected that those four HEs could inhibit L. monocytogenes growth at 12 °C, which represented to the abused refrigerator temperature. We investigated the antimicrobial effects of HEs 2, 4, 5 and 8 at three concentration levels (3.13, 1.56, 0.78 mg/mL) against a five-strain L. monocytogenes cocktail. The initial populations of L. monocytogenes cocktail in control and all treatment samples were 2.3 log CFU/mL. For control samples without any HE, bacteria population rapidly increased from 2.3 log CFU/mL (day 0) to 8.4 log CFU/mL by 4 days, and then increased to 8.8 log CFU/mL by day 7. After 7 days, bacteria population did not have further increase in number. For treatment samples, the growth of L. monocytogenes during refrigerated storage was dependent on the type of herb and HE concentration. In general, compared with control samples, lower bacteria populations (P < 0.001) were observed in all treatments except for the HE 8 at the concentration of 0.78 mg/mL (Tables 3-5).
At a concentration of 3.13 mg/mL (Table 3), HEs 2, 4, 5 and 8 reduced inoculated L. monocytogenes populations from 2.3 log CFU/mL to 0.2, 0.1, 0.7 and 0.5 log CFU/mL at day 11, respectively. Compared with positive control samples without any HE, each of four HEs had lower bacterial population (P < 0.001) on each day from day 1 to day 11. This result indicated that at the concentration of 3.13 mg/mL, all four HEs effectively reduced bacteria populations of L. monocytogenes cocktail at 12 °C.
Table 3: Least square means ± standard deviation of Listeria monocytogenes cocktail populations in inoculated Mueller-Hinton broth with each of four herb extracts at concentration of 3.13 mg/mL or deionized water (control), stored at 12 °C for 11 days (n=6).
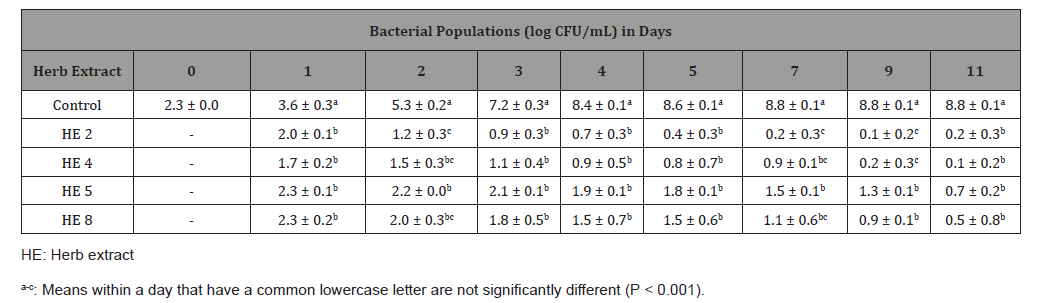
Table 4: Least square means ± standard deviation of Listeria monocytogenes cocktail populations in inoculated Mueller-Hinton broth with each of four herb extracts at concentration of 1.56 mg/mL or deionized water (control), stored at 12 °C for 11 days (n=6).
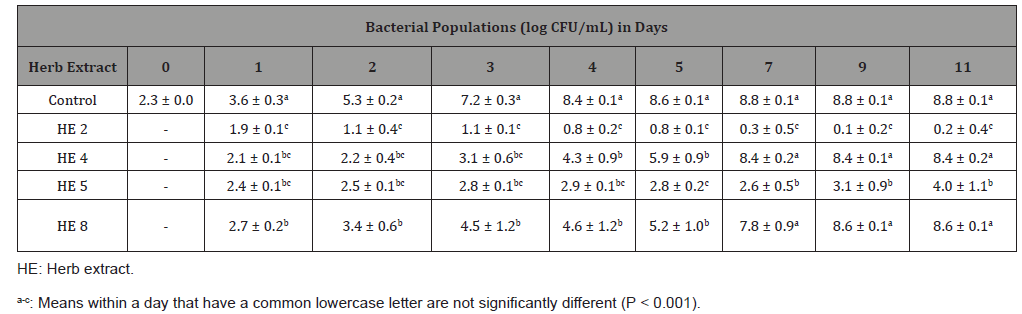
Table 5: Least square means ± standard deviation of Listeria monocytogenes cocktail populations in inoculated Mueller Hinton broth with each of four herb extracts at concentration of 0.78 mg/mL or deionized water (control), stored at 12 °C for 11 days (n=6).
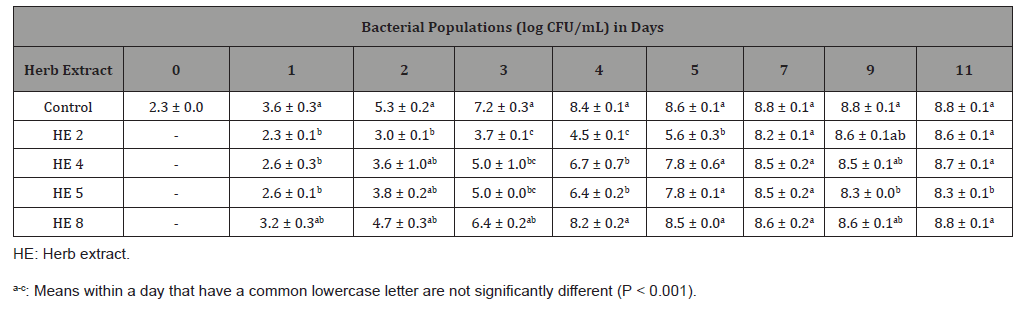
At the concentration of 1.56 mg/mL (Table 4), HE 2 reduced L. monocytogenes populations from 2.3 log CFU/mL to 0.2 log CFU/ mL at day 11, which was 8.6 log CFU/mL lower (P < 0.001) than the control. Although counts of L. monocytogenes in the sample with HE 5 increased from 2.3 log CFU/mL to 4.0 log CFU/mL at day 11, it was still 4.8 log CFU/mL lower (P < 0.001) than the control. However, compared with the control, HE 4 and 8 were lower (P < 0.001) in bacteria populations only up to 5 days. Therefore, antimicrobial effects of those four HE at concentration of 1.56 mg/ mL were ranked from the strongest to weakest as follows: HE 2 > HE 5 > HE 4 = HE 8.
Table 5 shows the inhibitory effects of each of the four HEs in MHB at the concentration of 0.78 mg/mL. Counts of the L. monocytogenes cocktail in the sample with HE 8 were not different (P > 0.001) from the control sample on each day from day 1 to day 11, indicating that HE 8 at a concentration of 0.78 mg/mL could not inhibit bacterial growth. Counts of samples with HE 4 or 5 increased from 2.3 log CFU/ mL to 6.7 and 6.4 log CFU/mL by day 4 respectively, which were lower (P < 0.001) than the control by about 2 log CFU/mL. After 4 days of incubation, the bacterial population of the sample with HE 4 were not different (P > 0.001) with the positive control sample on each day from day 5 to day 11. Although the sample with HE 5 did not show different (P > 0.001) in bacteria population with the positive control sample at day 5 and day 7, the population of HE 5 was 0.5 log CFU/mL lower (P < 0.001) than the control at day 9 and day 11. In addition, comparing with positive control samples, HE 2 slowed the microbial growth and reached to 5.6 log CFU/mL by day 5, which was lower than the controls for 3 log CFU/mL (P < 0.001). After 7 days of incubation, the bacterial population of the sample with HE 2 were not different (P > 0.001) with the control sample on each day from day 7 to day 11. In summary, at the concentrations of 0.78 mg/mL, HE 2 inhibited the microbial growth up to 5 days; HE 4 and 5 inhibited L. monocytogenes growth up to 4 days; HE 8 could not inhibit the microbial growth.
The demand for convenience foods such as RTE foods has increased in recent years. The majority of listeriosis cases are foodborne [22] and linked to the consumption of RTE foods which are contaminated with L. monocytogenes. Due to the high mortality rate of listeriosis, the U.S. Department of Agriculture and the FDA labels L. monocytogenes as an adulterant of RTE foods. Examples of RTE foods that support the growth of L. monocytogenes are milk, high fat dairy products, soft unripened cheese, cooked and raw seafood, deli-type salads, sandwiches, fresh-cut vegetable and fruits [23] and the processed meat which is under refrigerator conditions [24]. Although L. monocytogenes will continue to thrive at low temperature as 1.1 °C [25] the storage temperature and duration of refrigerated storage before consumption are important factors which reduce the risks of foodborne listeriosis [26]. The recommended refrigerator temperature is 40 °F (4.4 °C); however, abuse home refrigerator temperature can rise to more than 12 °C [26,27].
Previous published studies indicated that the inhibitory efficacies of plant-derived antimicrobials may be affected by temperature [28,29]. The results from Exp. 1 showed that HEs 2, 4, 5 and 8 exhibited inhibitory effects against each of five L. monocytogenes strains at 37 °C. However, in order to use those four HEs as food preservatives, they must be effective against L. monocytogenes under food storage conditions. In this experiment, inhibition efficacies of those four HEs were evaluated at 12 °C which represented the abused refrigerator temperature. As discussed above, at concentrations of 1.56 and 0.78 mg/mL, HEs 2, 4, 5 and 8 inhibited growth of a five-strain L. monocytogenes cocktail at abuse refrigeration temperature of 12 °C, except herb extract 8 at the concentration of 0.78 mg/mL. At a concentration of 3.13 mg/mL, these four HEs reduced cell populations in a range of 2.2 to 1.6 log CFU/mL at 11 days. In a previous study, thyme essential oil showed the inhibitory effect against L. monocytogenes cocktails at 10 °C up to 12 days in minced beef [30]. HEs 2, 4, 5 and 8 were also expected to be developed into food preservatives for inhibiting and/or reducing foodborne L. monocytogenes. For example, those four HEs could be added to RTE foods as supplements or incorporated into food packaging materials to control L. monocytogenes growth. Further experiments should be conducted to determine the inhibitory effects and reductions of those four HEs in food products. In addition, since HEs carry specific odor, palatability of the food applied with HEs should be evaluated by sensory panel.
Conclusion
In summary, HEs 2, 4, 5 and 8 exhibited inhibitory effects against L. monocytogenes at 37 °C in a range of MIC between 5 - 50 mg/mL. HE 4 reduced cell populations of each selected strain ranged between 0.38 -
0.91 log CFU/mL after 30 min treatment at 37 °C. In addition, at concentrations of 1.56 and 0.78 mg/mL, HEs 2, 4, 5 and 8 inhibited growth of a five-strain L. monocytogenes cocktail at 12 °C, except the HE 8 at the concentration of 0.78 mg/mL. At a concentration of 3.13 mg/mL, these four HEs reduced cell populations in a range of 2.2 to 1.6 log CFU/mL at 11 days. For their practical application, further experiments should be conducted to determine the inhibitory effects and reductions of those HEs in a variety of food products. In addition, palatability of the foods which applied with HEs should be evaluated by sensory panel
To read more about this article...Open access Journal of of Agriculture and Soil Science
Please follow the URL to access more information about this article
https://irispublishers.com/wjass/fulltext/antimicrobial-effects-of-herb-extracts.ID.000590.php
To know more about our Journals...Iris Publishers
To read more about this article...Open access Journal of of Agriculture and Soil Science
Please follow the URL to access more information about this article
https://irispublishers.com/wjass/fulltext/antimicrobial-effects-of-herb-extracts.ID.000590.php
To know about Open Access Publishers





No comments:
Post a Comment