Authored by Rosane Aguiar da Silva San Gil*,
Abstract
The predisposition of pharmaceutical solids to crystallize in various forms can lead to differences in their physical and chemical properties. The study of polymorphism is an important step in drug development because it causes a relevant physical and chemical characteristic in both the API and the formulated drug. NMR is one of the most versatile techniques for structural characterization. In addition, Solid State NMR is important for the study of organic and inorganic compounds, both for structural determination and for the use of samples, bonding types and geometry, as well as for analytical studies and their applications in a wide range of areas, including drug studies, such as pure API or pharmaceutical formulations and excipients. In this mini review, there are some examples about its use for studying polymorphism in pharmaceuticals.
Keywords: Solid state NMR; 13C CPMAS; Polymorphism; Pharmaceuticals
Mini Review
NMR is one of the most versatile techniques for structural characterization. In addition, NMR is important for the study of organic and inorganic compounds, both for structural determination and for the use of samples, bonding types and geometry, as well as for analytical studies and their applications in a wide range of areas, including drug studies, such as pure IFA or pharmaceutical formulations and excipients. All types of solid material can be studied by NMR, including crystals and powders, amorphous materials such as glass and rubber, superconductors and metals [1]. The Hamiltonian that describes the interactions present in a sample containing nuclear spin nuclei (I), consists of a sum of Hamiltonians related to the magnetic and electrical properties of the sample being analyzed [2], as shown EQUATION 1:

Where Hz is Zeeman Hamiltonian, HRF radiofrequency Hamiltonian, HJ scalar Hamiltonian, HD dipolar Hamiltonian, HDQ chemical shift Hamiltonian and HQ quadrupolar Hamiltonian. Zeeman’s interaction between nuclear spin and the applied magnetic field induces the precession in the Larmor frequency ωo, which is defined by the nucleus and the external field strength. Magnetic fields as high as 21 T are in use, producing 900 MHz Larmor frequencies for hydrogen. Internal interactions observed include those of chemical shift, and scalar couplings. In addition, solid state NMR [1,2] can detect chemical shift anisotropies, magnetic dipole and electric quadrupole interactions, which are only observed indirectly in solution NMR. The most remarkable difference between the spectra obtained in solid NMR and in solution NMR is that in solid form the spectra show very broad signals. This difference is a function of the strong dipolar coupling interactions and the solid-state chemical shift anisotropy, which in the liquid state is averaged to its isotropic value or eliminated by molecular motion. Both dipolar coupling and chemical shift anisotropy are geometry dependent phenomena. The first gives relative orientation between two dipolar nuclei under study, while the second is a function of the orientation of the nucleus in relation to the static magnetic field [3].
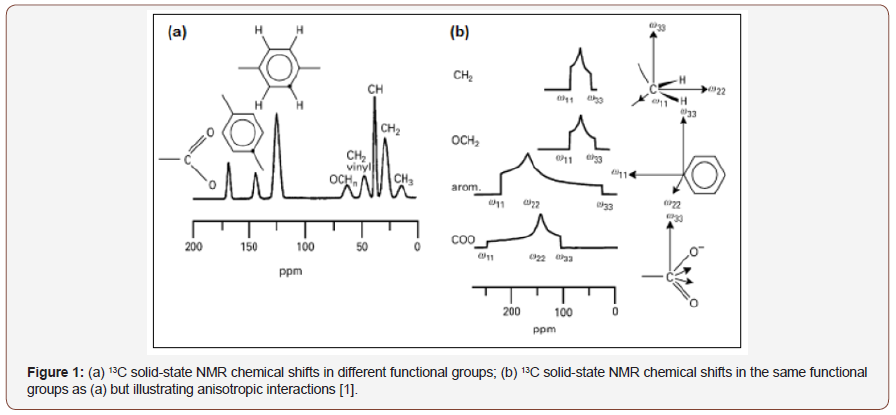
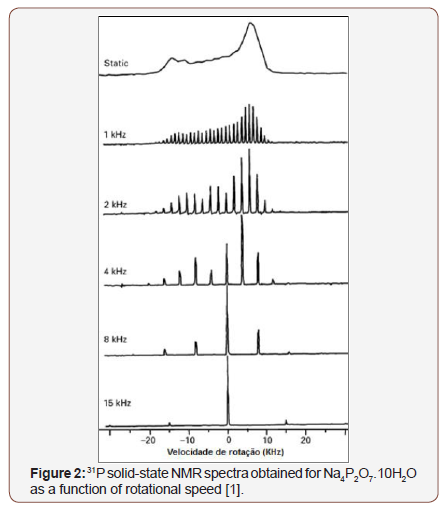
The development of the techniques of magic angle rotation - MAS [4], high-power decoupling [5] and cross-polarization - CP [6] allowed obtaining high-resolution NMR spectra in solid form. The implementation of several pulse sequences, such as TOSS (Total Suppression of Sidebands) that suppresses sideband signals [7], the dipolar dephasing technique (DD) for signaling non-proton carbons [8], and two-dimensional experiments such as PASS-adjusted spinning sidebands [9], allowed solid state NMR to be applied more broadly. The Figure 1 illustrates the solid state NMR spectrum, where the molecular orientation dependent interactions were eliminated by positioning and rotating around the magic angle (MAS) and the spectrum becomes similar to an NMR spectrum in the Figure 1, where dipolar and quadrupolar anisotropic interactions are maintained [1]. The most important technique for improving the resolution of solid-state NMR spectra is the sample positioning at 54.74° and the rotation at velocities of the order of magnitude of dipolar interactions and chemical shift anisotropies. Typically, for nuclei like 13C, 31P and 29Si that have narrow chemical shift ranges, rotational speeds between 5 and 10 KHz, are sufficient to nullify dominant anisotropic interactions, as can be seen in Figure 2 [1,2]. On the other hand, the MAS technique significantly improves the resolution of spectra when coupled with the CP technique, and an improvement in sensitivity can be achieved. In the CPMAS technique, first magnetization of abundant species (1H) is done and then this magnetization is transferred to less abundant species, such as 13C and 15N, by dipolar interaction. The least abundant species are detected while uncoupling the most abundant species. The theoretical sensitivity gain is the ratio of the Larmor frequencies of the two spins, e.g. up to 4 to the 1H-13C [1,2] (Figure 1,2).
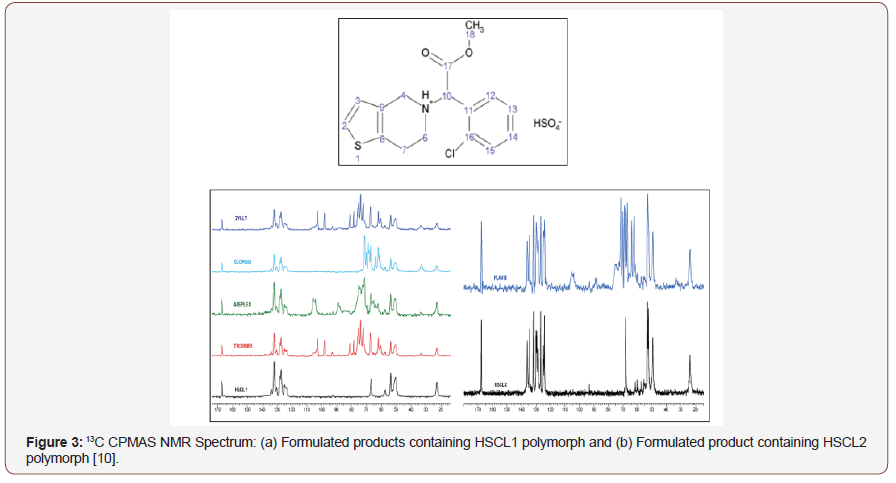
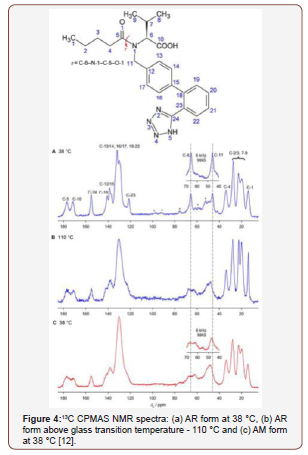
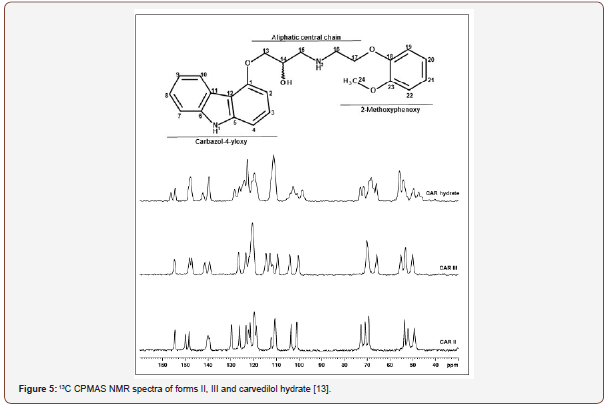
Solid-state NMR has been widely used in the identification of API polymorphs and in the characterization of pharmaceutical formulations, as well as in stability evaluation and drug-excipient interaction. In 2015, PINDELSKA and colleagues [10] used solid-state NMR for the analysis of clopidogrel hydrogensulfate polymorphs (HSCL), the antiplatelet agent, and the world’s second-best selling drug. The solid dosage forms of the drug were evaluated, and the methodology was used to identify which polymorph was present in the commercial formulation. HSCL exists in different polymorphic forms, but only one of the two may be present in the final formulation, the HSCL1 polymorph or HSCL2. The standard spectra of the two polymorphs were obtained and compared with the 13C CPMAS spectra of the different formulated products. Polymorphic forms were easily identified in pharmaceutical formulations by comparing the 13C solid-state NMR spectra (Figure 3). AGUIAR et al. (2016) have used a combination of 13C solid state NMR and GIPAW-DFT computed isotropic calculation to solve ambiguities in 13C chemical shifts of 6-aminopenicilanic acid (6-APA). This is an intermediate in the industrial preparation of beta-lactam penicillin’s [11]. SKOTNICKI et al. (2016) characterized two forms of valsartan IFA, an antihypertensive drug, by solid-state NMR [12]. From the 13C CPMAS NMR spectra it was possible to distinguish between the AR form (as received from the manufacturer with low crystallinity) and the AM form (completely amorphous), observing the region between 40 and 70 ppm that was distinct for the two forms analyzed (Figure 4). REZENDE et al. (2016), who studied polymorphs from carvedilol API (CAR), an antihypertensive, reported another recent work about solid-state NMR polymorphism identification [13] (Figure 3,4).
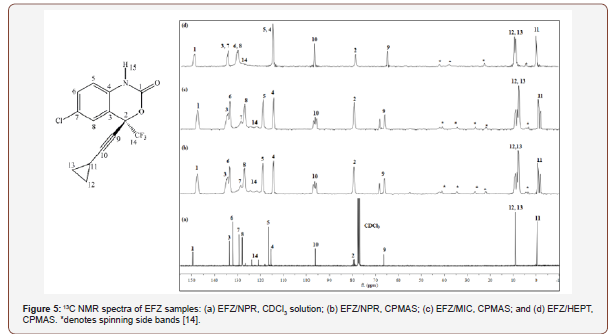
was possible to characterize by 13C CPMAS NMR three polymorphic forms of the drug: form II, III and hydrate Figure 5. Solid-state NMR spectra signals were performed by the combination of NMR techniques and ab initio calculation.In the same year (2016), Sousa and coworkers studied polymorphism in efavirenz (EFZ), an antiretroviral drug [14]. In this study, the authors could be found, by solid-state NMR, the difference between EFZ used in the medicine formulations and the polymorphic form obtained by EFZ crystallization in heptane solvent. 13C CPMAS NMR spectra Figure 6 of the EFZ/MIC and EFZ/HEPT showed 2 spectral regions, which present clear differences like signals multiplicity and intensity ratios of the signals. The first region of comparison is 60-100 ppm, and the second region of interest is the cyclopropyl group between -2.0 and 10 ppm (Figure 5,6).
Recently Solid-state NMR has been used combined with DFT calculations, to study polymorphism in dexamethasone acetate, by SILVA et al. in 2018 [15], inpharmacy samples of melxican [16] by ROMANI et al. (2018) and for evaluation of piracetam by Szeleszczuk et al. (2019). Piracetam is a popular nootropic drug, widely used in the treatment of age-associated mental decline and disorders of the nervous system such as Alzheimer’s disease and dementia exists under normal pressure in three polymorphic forms (P1, P2 and P3) of different stability. The authors studied the relative stability of piracetam polymorphs depending on the temperature by using the solid-state NMR spectroscopy combined with ab initio DFT calculations. The results showed that DFT calculations can be used not only to obtain the NMR parameters but also to predict the influence of the temperature on the stability order of the polymorphic forms of molecular crystals [17]. From the examples given it can be said that solid-state NMR offers a range of relevant information to basic scientific research or industry specific requirements unparalleled by any other technique.
To read more about this article...Open access Journal of Pharmacy & Pharmacology Research
Please follow the URL to access more information about this article
To know more about our Journals...Iris Publishers





No comments:
Post a Comment