Authored by Nevin Yılmaz*,
Introduction
Globally, about 290 000 people died from hepatitis C (HCV) in 2019, mostly from cirrhosis and hepatocellular carcinoma (HCC). Less than a decade, direct-acting antivirals (DAAs) replaced interferon-based therapies in treatment protocols and sustained virologic response (SVR) rates exceeded 95%. However, it is not clear yet whether HCV eradication by DAAs can reverse fibrosis or cirrhosis substantially, reduce the risk of developing HCC in the long term. In this review was aimed to summarizes the evidence for reversibility or progression of fibrosis following HCV clearance by DAAs, subsequently its impact on developing de nova HCC. For this purpose, cohorts in which patients achieved SVR, have paired liver stiffness measurements obtained by transient elastography were reviewed and results discussed.
Keywords: HCV infection; Hepatocellular carcinoma; Fibrosis; Sustain virologic response; Direct-acting antivirals; Liver stiffness
Introduction
Globally, an estimated 58 million people have chronic hepatitis C virus (HCV) infection and according to the WHO reports, approximately 290 000 [Range: 230 000–580 000] people died from hepatitis C in 2019, mostly from cirrhosis and hepatocellular carcinoma (HCC) [1]. In general, 10–20% of chronic HCV advance to cirrhosis over a 20–30 year period and once cirrhosis is established, HCC develops at an annual rate of 1% to 5% [2]. Considering that approximately 90% of HCV-related HCC cases develop on the basis of cirrhosis, HCV likely promotes tumorigenesis through repeated damage, regeneration, and fibrosis [3]. In addition, HCV induces epigenetic alterations which are risk factors for HCC, are maintained after HCV clearance [4-6].
Fibrosis, of which hepatic stellate cells are key source, is a wound-healing response to chronic liver injuries and results from an imbalance between extracellular matrix synthesis and degradation. Although fibrosis are being reversed in several types of liver disease, ongoing fibrosis frequently resulted with cirrhosis [7, 8]. Based on the interferon (IFN) - based therapy experiences, HCV eradication reduces the risk of HCC independently(independent) of the grade of fibrosis [9]. In 2014, direct-acting antivirals (DAAs) replaced INFs in treatment protocols and sustain virological response (SVR) rates exceeded 95% subsequently, a 71% reduction in HCC risk was reported [10]. However, it is not clear yet whether HCV eradication by DAAs can reverse fibrosis or cirrhosis substantially; reduce the risk of developing HCC in the long term. The fact that DAAs have been in use for less than a decade and the lack of follow-up biopsies after SVR has made reliable confirmation of fibrosis changes difficult. Recently, widely use of validated non-invasive tests have made it easier to follow fibrosis changes, though not as much as liver biopsy. In particular, liver stiffness measurement (LSM) by transient elastography (TE) is an accurate, indirect, noninvasive method to assess liver fibrosis grade [11].
In this review was aimed to summarize the evidence for reversibility or progression of fibrosis following HCV clearance by DAAs subsequently, its impact on developing de nova HCC. For this purpose cohorts in which patients achieved SVR, have paired (baseline and follow-up) liver stiffness measurements obtained by TE were reviewed. Availability of HCC data in the same study and at least 2-years follow-up were the other criteria. To ensure homogeneity, studies involving history of HCC, human immunodeficiency virus (HIV), hepatitis B virus (HBV), transplantation, or other liver diseases were not reviewed. Searches in English were performed using PubMed, for HCV, HCC, hepatic fibrosis, regression, liver stiffness, DAA and SVR.
What are the results of clinical studies on this subject?
In our searches, we were able to find a few studies that included our criteria and whose data could be extracted. Genotypes, baseline and follow-up fibrosis changes, as well as new HCC cases developed during follow-up were summarised in this section and the (Table 1).
Table 1:Summary of Studies; Liver Stiffness Changes at different time points of follow-up in patients with SVR.
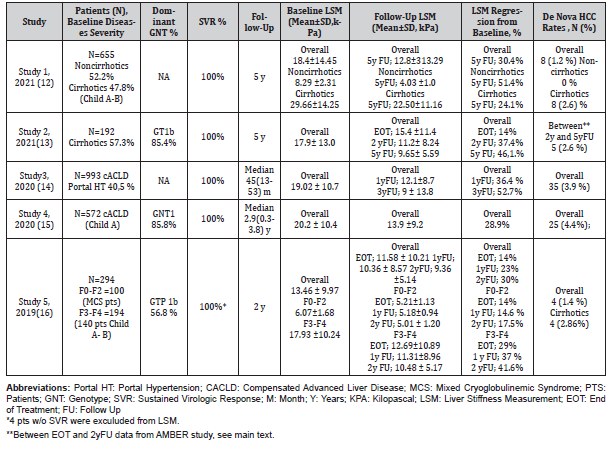
Study 1
One of the most recent study is from Egypt. Zakareya, et al. [12] studied 655 patients (48% cirrhosis; Child A- B) who were treated with DAAs and successfully achieved SVR. End of the 5-years follow-up (yFU), in patients without cirrhosis the mean overall decline in LSM was 4.26 kPa (kilopascal), representing about 51.4% of the baseline value and in cirrhotic patients, there was 7.16 kPa declines representing 24.1% of the baseline value. According to the study, cirrhosis have regressed to lower fibrosis stages in 87 patients (27.7%) and regression to lower fibrosis stages was more prominent in F2 patients. At 5th year, insignificant progression was reported in only 4.6% of patients with F3 fibrosis (mean 1.7kPa increase) and in 8.9% of cirrhotic patients (mean 2.1kPa increase). The authors concluded that mild fibrosis (F0–F2) usually improves shortly after treatment (at SVR and 1-year), while advanced fibrosis (F3–F4) take a longer time to regress to lower fibrosis stages. De nova HCC developed in 8 cirrhotic patients (2.6%) and 2 of them had a progressive pattern of LSM, while the remaining 6 had a regressive pattern.
Study 2
Flisiak, et al. [13] from Poland analyzed a total of 192 patients out of 209 of the primary AMBER study 5y after treatment. Liver stiffness results at baseline, EOT, 2yFU, and 5yFU were available in 78 patients. The mean LSM of 78 patients decreased significantly from 17.9 kPa at baseline to 9.65 kPa at 5 yFU representing 46.1% of the baseline. The overall mean decrease from baseline in LSM was 2.5 kPa at EOT, 6.7 kPa at 2yFU and 8.25kPa at 5 years after treatment. The increase in LSM during 5yFU was demonstrated only in 4 patients and did not exceed 2.1 kPa. A total of 12 cases of HCC was diagnosed during the whole duration of follow-up, 7 within the first 2 years, and 5 in the subsequent 3 years with annual rates of 1.7 % and 0.9%, respectively. Three out of 12 had no cirrhosis at baseline. However, 3 of 7 HCC diagnosed between EOT and 2yFU (primary AMBER study) were recurrent HCC. Hence the annual de nova HCC rate can be interpreted as 0.96% between EOT and 2-years follow-up period.
Study 3
Lopez, et al. [14] from Spain followed 993 compensated advanced chronic liver disease (cACLD) patients with SVR (40.5% portal hypertension) for median 45 months (Range= 13-53) after EOT. The overall mean decline from baseline in LSM was 6.92 kPa at 1yFU and 10.02 kPa at 3yFU representing 36.4% versus 52.7% of baseline reduction, respectively. De nova HCC developed in 35 (3.9%) patients and the mean LSM was 28.28 ± 14.91 kPa while it was 19.12 ± 10.59 kPa in non-developing patients. The authors concluded that albumin and liver stiffness (LS) at the initiation of DAA therapy and the percentage of change of LS at one-year after EOT were independent predictors of HCC development.
Study 4
Pons, et al. [15] from Spain followed 572 Child A patients with SVR for median 2.9 (range 0.3-3.8) years. After 1yFU LSM was examined in 554 patients. Overall mean baseline LSM was 20.2 kPa and at 1yFU reduced to 13.9 kPa representing 28.9% of the baseline decline in patients with paired measurements. HCC occurred in 25 patients (4.4%) being the incidence rate 1.5/100 patient-years (PY) and the median time for HCC occurrence was 1 year (range 0.4-3.3 years). In the cohort 212 patients had baseline LSM ≥20 kPa and of them 13 (6.1%) presented HCC, while HCC occurred in 12 patients with baseline LSM <20 kPa (3.3%), being these differences not statistically significant. However, patients with LSM <10 kPa at follow-up (HR 0.33; 95% CI: 0.11-0.96, P=0.042) were found to have a low risk of presenting HCC.
Study 5
Stasi, et al. [16] studied 294 chronic HCV patients for 2 years after EOT. In the study, the F0-F2 and F3-F4 patients were evaluated separately. All F0-F2 patients (N=100) had the mixed cryoglobulinemic syndrome (MCS) disorder and in the F3-F4 group 140 patients had Child A or B cirrhosis. At 2 yFU, in F0-F2 group the mean overall decline in LSM was 1.06 kPa, representing about 17.5% of the baseline value and in F3-F4 group, there was 7.45 kPa decline representing 41.6 % of the baseline value. The changes at other times points are summarized in the Table. De nova HCC developed in 4 patients all had cirrhosis at baseline. In this study, the authors stated that the extent of fibrosis is an important negative predictor of HCC development.
Interpretation of the presented studies
Longitudinal assessment of liver fibrosis following SVR
In the all study groups overall mean baseline LSM values were ≥ 12kPa. Considering the all groups, the mean LSM improvement (regression from baseline) at 5y, 2-3 y, 1y, and EOT were 38.25%, 37.25%, 29.7%, and 14%, respectively. These results indicate that the improvement in LSM begins during EOT, increases in the first 1 year, and tends to slow down 3 years after EOT. In addition, comparing F0-F2 with F3-F4 of study 5 [16], improvement in LSM in the F0-F2 subgroup was almost 50% lower at EOT, 1 year and 2 years (Table 1). End of the 2yFU, mean delta (Δ) was 0.89 kPa vs 6.62 kPa in the F0-F2 and F3-F4 subgroup, respectively. These findings suggest that mostly necroinflammation regress in the first year of SVR, and the percentage of regression in this time-frame might be correlated with the degree of inflammation.
Given the regression of fibrosis is a relatively slow process compared with the inflammation, histologically real regression of fibrosis takes longer time to be degraded [7,9]. In study 1, 27.8% of patients with cirrhosis regressed to lower stages at 5 years [12]. Similarly, reversal of cirrhosis reported in 21.8% of F4 patients over a follow up of 2 years by Shiha, et al. [17], Transient elastography is the most widely used and validated technique. One of its disadvantages is that it cannot adequately differentiate intermediate stages of fibrosis in patients with chronic liver disease [11]. There are also additional factors that may exert an impact on liver stiffness, e.g., inflammation, blood flow, and portal pressure [18]. Notwithstanding, TE has good-to-excellent accuracy in diagnosing advanced fibrosis and cirrhosis following SVR [11,18]. There are few HCV studies comparing LSM and biopsy results regarding fibrosis changes. In a study by Mauro, et al. [19], a 50% reduction in baseline LSM was accurately predicted in 55% liver biopsy with a positive predictive value (PPV) of 78% and a negative PV of 44%. At the same study, baseline LSM with cut off <21 kPa, was defined as an independent predictor of fibrosis regression [19].
Fibrosis progression following SVR
Slight increases in liver stiffness at 5-year follow-up were reported by 2 studies [12,16]. In study 1 [12], insignificant progression over 5 years was reported in 4.6% of patients with F3 fibrosis and 8.9% of cirrhotic patients. The mean increases were 1.7kPa and 2.1 kPa, respectively. In study 2 [13], an increase not exceeding 2.1kPa was reported in 4 patients. However, in study 5 [16] which has lower overall baseline LSM and shorter follow-up, increasing in stiffness was not observed at 2-years follow-up.
Time elapsed after SVR and initial fibrosis stages most likely predict progression of fibrosis in these patients. Ongoing co-factors such as alcohol and metabolic syndrome may affect this process [2].
Different from the results presented, Shiha, et al. [17] reported that 11.4% of patients in their cohort progressed from F3 to F4 stage over 2 years of FU. The reference range of LSM was slightly different in the study and obese patients were included to the cohort. Similarly, Pietsch, et al. [20] reported 17 % fibrosis progression over 96 weeks of FU by using a different reference range. IFN-based studies also showed fibrosis progression after SVR in a small group of patients (1-14%) [2].
De nova HCC rates in this dynamic process following SVR
In these six studies which overall liver stiffness improved after SVR, denova HCC rates ranged from 1.2% to 4.7% at 5yFU and 1.4%-4.4% at 2-3yFU. In study 1 [12], regression type fibrosis was reported in 6 of 8 HCC cases developed in patients with cirrhosis. In study 3, albumin and LSM at the initiation of DAA therapy and the percentage of change of LSM at one-year after EOT were reported as an independent predictors of HCC development by Lopez, et al. [14]. Additionally, in study 4 [15], patients with LSM <10 kPa at follow-up were found to have a low risk of presenting HCC, There are few similar published studies. Morisco, et al. [21] reported that baseline liver stiffness ≥20kPa was an independent predictor of HCC and the incidence rate of denova HCC was 1.6 per 100 person years (PY) in their cirrhotic-SVR cohort. Similarly, baseline and SVR24 LSM values were defined as independent predictors of HCC by Ogasawara, et al. [22]. The annual denova HCC rate in the first 4 years was 1.5% in their SVR cohort and the median interval between SVR and HCC detection was 1.7 (range, 0.6-3.2) years [22].
Shiha, et al. [17] reported that the incidence of HCC was 2.917/100 PY vs 0.664/100 PY in F4 and F3 patients, respectively in their SVR cohort. Also, the authors concluded that regression of fibrosis is associated with decreased incidence of HCC. In one of the largest cohort from the United States (N= 19,518 with SVR), reported rates of denovo HCC were 1.82 vs 0.34/100 PY in patients with cirrhosis and without cirrhosis respectively [23]. These data suggest that HCV patients with SVR still at risk for denova HCC even in the first 3 years of SVR that fibrosis regression is higher. A few more studies have reported an association between denovo HCC and LSM [24]. Recently, pre-treatment LSM and ΔLSM were included in a predictive model [14] but, still need prospective validation. Limitations in this review; long-term follow-up results of DAAs have not been published yet, and some data were not suitable for analysis.
Conclusion
These data show that fibrosis regression detected by TE begins during EOT and tends to slow down after three years. Early changes might indicate the regression in necroinflammation but, reversal of cirrhosis is detectable before three years. Despite viral clearance, progression of LSM may occur in a minority of patients, especially in advanced cases. Although LSM improvements are overstated when compared to histologic staging, baseline stiffness and changes in stiffness following SVR may predict the risk of denova HCC. The risk of HCC is present even in the early years after SVR, especially in those with advanced fibrosis or cirrhosis. In these patients, surveillance should be continued. The investigation of HCC predictors based on transient elastography may better optimize surveillance strategies in these patients.
To read more about this article...Open access Journal of Gastroenterology & Hepatology
Please follow the URL to access more information about this article





No comments:
Post a Comment