Authored by Le Phuoc Cuong*,
Abstract
In recent years, the micropollutants are increasingly detectable in the aquatic environment and endanger the human health even on shortterm. The impacts of pharmaceuticals and personal hygiene care products and chemicals in the aquatic environment is not clearly understood yet. The drug residues, household chemicals and pesticides are micropolluting components in our waters. The paper deals with different fourth stage wastewater treatment technologies including the advanced oxidation processes for the removal of micropollutants. The micropollutant removal efficiencies are studied and compared in light of the different technological solutions.
Keywords: Removal; Micropollutants; Human health; Oxidation process; Wastewater
Introduction
The task of wastewater treatment is to clean the contaminated waters which are discharged into the environment after treatment. The surface waters must reach a good ecological status according to the Water Framework Directive. It is important not to load the recipients with contminating materials and nutrients which are heavily decomposable. The goal of the sewage treatment is to lower the anthropogenic effects in the hydrological system to the lowest possible level. Thus, the task is typically to reduce the concentration of nutrients, to remove the dissolved oxygen consuming substances and to remove the accumulated organic and inorganic substances from wastewater [1,2].
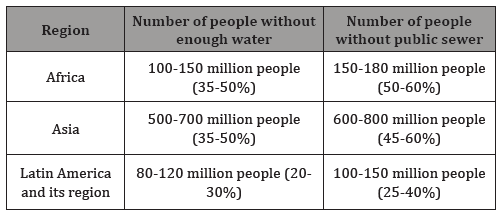
It is clear that in the 21th centur the protection of human health, the implementation of the concept of sustainable development and the protection of the ecosystem continue to play an important role. In many articles [3,4] it is emphasized that urbanization is increasing and the associated wastewater treatment poses a huge burden on the environment [5]. Among the readers of the new scientific results there are only few who have learnt from their own experience the social, economic and environmental problems of water scarcity. However, in many regions Table 1, there are millions of people who lack the water supply or the comfort of the public sewage system. With the introduction of the third stage of the sewage treatment, numerous problems have been resolved. However, there are still many issues that need to be solved. These questions raise the following tasks: the removal of anthropogenic micropollutants,which are often persistent. The WFD guidelines stipulate, the achievement and maintenance of good ecological status, wherever is possible, and the healthy drinking water supply. To solve these problems, many laboratories, research institutions and industrial companies deal with the performance and effectiveness of wastewater treatment technologies (Table 1), [6].
Table 1: Number and proportion of urban dwellers lacking adequate water supply and sewerage (2000) [4].
Micropollutants
Micropollutants are those substances that can be found in waters in magnitude of μg/l and reduce or eliminate the conditions of life processes in natural living waters. In this way the usability of waters as potable waters is drastically reduced or eliminated. Most of these micropollutants entering into living waters have persistent properties. One part of these contaminating materials can be removed in municipal sewage treatment plants, however significant part of these cannot be eliminated in the conventional wastewater facilities [A26]. These micropollutants can be organic micropollutants, inorganic micropollutants, drugs and drug residues, medicines, pesticides, etc. [7]. Therefore a fourth-stage treatment line is necessary to be considered in the wastewater treatment. In recent years, these micropollutants are increasingly detectable in the aquatic environment, as reported in the literature [8]. Christian and Thomas’ work is a good summary of the effects of pharmaceuticals and personal hygiene care products and chemicals in the aquatic environment. Their work suggests that drug residues, household chemicals and pesticides significantly pollute the surface waters. The source of these pollutants can be effluents, untreated waters, drain offs and flood streams [9]. Conventional sewage treatment plants receive a wide spectrum of such pollutants, which cannot be completely removed by conventional treatment procedures, so these materials accumulate in the recipients, as described by Yoon et al. [10].
The concentration of these pollutants is increasing, and today they are increasingly detectable in surface waters. These impurities typically exhibit significant toxicity even at low concentrations. Pollutants with hydrophilic properties also pose a threat to species of aquatic ecosystems [9]. In many cities, it can be observed that the water resources are contaminated in certain extent with effluents, so micropollutants entered into the drinking water bases. Thania and his colleagues dealt with Mexico City’s drinking water base and they identified 17 organic micropollutants whose concentrations were over the limit values, and it was proven that those derived from anthropogenic sources [11]. Yunlong Luo et al. in their 2014 article concluded that the novel tasks of wastewater treatment include maximizing the efficiency of the micropollutant removal during the optimization of sewage treatment plants. Some pollutants cannot be removed at all, others can only be party removed in the traditional wastewater treatment plants. It is mentioned that highly efficient methods, like advanced oxidation processes (AOP), adsorption processes and membrane separation procedures are all suitable for micropollutant removal, but these additional technological steps significantly increase the operating costs of the treatment facilities [7]. According to Maria Gavrilescu et al., these pollutants exhibit a serious challenge for the environment and human health in the future. They also pointed out that the operational treatment technologies cannot handle the new challenges [12].
Pesticides
Pesticides are mixtures of substances to protect plants and thus increase the agricultural productivity. Pesticides are vital elements of the modern agriculture, they play a significant role in highquality production. However, the long-term use of pesticides could exhibit a negative impact on the environment and human health [13]. A significant part of these adverse effects is due to the fact that they are being dispensed in inappropriate doses to agricultural areas or that inappropriate pesticides are used [14]. Mustapha and his associates also revealed a significant fact that in many countries banned pesticides are used [15]. The presence of these components in the environment is a potential source of danger [16].
Pharmaceutical Residues
The presence of pharmaceutical residues and hormones in sewage and surface water has been confirmed in several articles [17]. As the population increases, the use of medicines increases proportionally. In this way the presence of environmentallyunfriendly substances significantly increases in the environment [18]. Consequently, wastewater treatment plants will be heavily burdened with pharmaceutical residues. In the effluent of conventional sewage treatment plants, the concentration of drug substances also increases, which also accumulates in surface waters and accumulates due to their high biological stability [19]. The most frequently detected drug substances in wastewater are the following according to Bush’s work [20]:
Inflammatory and analgesic agents: paracetamol, acetylsalicylic acid, ibuprofen, diclofenac,
Antidepressants: benzodiazepines,
Antiepileptics: carbamazepine,
Lipid-lowering drugs: fibrates
β-blockers: atenolol, propanolol, metoprolol
Antibacterial drugs: antihistamines (ranitidine, famotidine)
Antibiotics: tetracyclines, macrolides, β-lactams, penicillins, quinolones, sulfonamides, fluoroquinolones, chloramphenicol, imidazole derivatives
Other substances: cocaine, barbiturates, methadone, amphetamines, opiates, heroin and other narcotics [20].
These compounds and their metabolites burden the sewage treatment plants. If the sewage treatment plants cannot remove the pollutants, the pollutants enter into the recipients, i.e. into the surface waters. The presence of these substances has a negative impact on the quality of the effluents. Therefore, continuous and targeted monitoring programs can positively contribute to solve this problem, however, additional mitigation measures are necessary.
Processes for the Removal of Micropollutants
Most of the micropollutants are not biologically decomposable or are very difficult to be broken down in the biological wastewater treatment units. For this reason, the micropollutants appear in the recipients unchanged [6]. There are a number of novel technological solutions for the removal of the micropollutants, and a brief summary on these will be given in this paper. Depending on the properties of the micropollutants, the coagulation and flocculation processes can be used to remove some or all of these compounds. From the work of Wray and Andrews, membrane separation processes are not always suitable to withhold the various organic micropollutants with good operational safety. The membrane separation technique can be efficiently combined with coagulation pretreatment [21]. Also due to the chemical and physical properties of the micropollutants those can accumulate in the the wastewater sludge. They are bound to the sludge matrix by chemical or physical adsorption. It is mentioned that during the biological processes the efficiency of the removal is influenced not only by the degradation but also by the sorption processes [22]. The procedures already referred to as fourth stage wastewater treatment are currently installed into the wastewater treatment train, however, these processes have not yet been widely applied in many countries. These proedures are used e.g. in Germany, Switzerland. Stefanos et al., discloses five different oxidation processes and three different post-treatment processes for Swiss treatment plants [23].
Micropollutants in Sewage Treatment Plants
The biological sewage treatments with present technologies are not suitable for removing the micropollutants efficiently. Micropollutants are specific for treatment, and there is no uniform process for their removal due to their different properties as described by Yunlong Luo et al. [7]. A number of articles and reports have been published in this area [24-33], Figure 1. summarizes the relative frequencies of the various AOP techniques used in the European Union between 2004 and 2014. The assessment took into account the groups of substances stipulated in Directive 2013/39 / EU [34], (Figure 1).

Several papers have been published over the last decades on the effectiveness of high efficiency oxidation processes for the removal of micropollutants. Giannakis et al. studied five different oxidation methods for micropollutant removal. The procedures studied were as follows: UV-C, UV-C/H2O2, Solar, Fenton, photo-Fenton procedures. It was found that the UV-C is the most efficient when the pretreated sewage was treated with MBBR (Moving Bed Biofilm Reactor). technology. Even after 10 min treatment the removal efficiency was around 50%. The treatments also exhibited similar results after 10 min of UV treatment, but there were significant differences in the performance after 30 min. After 30 min, the UVC oxidation process showed 70 % removal efficiency after coagulation, and 80% removal efficiency was reached after MBBR purification [23]. The UV/H2O2 process proved to be useful after the coagulation process. The MBBR process was the most effective in micropollutant removal. In case of MBBR after 5 min treatment 85-98 % of the micropollutants were removed, while after 10 min treatment 100% removal efficiency was achieved. It was concluded that the current methods used in wastewater treatment significantly affect the maximum removal efficiency achieved with post-treatment technologies. In the fourth stage of sewage treatment, not only the oxidation processes take place. Adsorption processes are also important to be considered. The oxidation processes combined with adsorption technique have reached outstanding results in the removal of micropollutants. Salvatore et al. studied the removal efficiency of the adsorption combined with high efficiency oxidation process to remove nitrophenol from waste water and a removal efficiency of 70 % was achieved [35].
Oxidation Processes
Ozonic oxidation processes are widely used in sewage treatment [36]. Ozone is not stable in an aqueous environment. There are several different solutions to mix the gas-water phases in order that the ozone can oxidize the micropollutants in the water. In the first step free radicals are formed when the ozone is decomposed and reacts with the organic compounds. Oxygen, odor-causing compounds, color-influencing compounds, volatile substances, humic substances, aromatic compounds [37] can easily be oxidized in this way. Advanced oxidation procedures (AOP) have also been extensively studied. According to Riberio et al. the chemical oxidation processes destruct the organic microcontaminants and majority of the complex compounds. During these processes hydroxyl radicals are formed which oxidize the organic pollutants. The products of the oxidation are CO2, H2O and inorganic ions [24]. Ozonization can be used in post-cleaning processes. The reason for this is that the chemical oxidation reduces the concentration of compounds which cannot be removed in the biological units or adsorptive methods. Ozone rapidly reacts with nitrite, so full nitrification takes place which is a prerequisite for the effective ozonation. Additional procedures attached to the conventional sewage treatment technologies to remove the organic micropollutants increase the energy needs. As a result, their environmental and economic impacts are significant. Table 2. summarizes the results of several studies on the energy demands of ozonization. The results of Danièle Mousel et al. show that the ozonic oxidation process used in sewage treatment plants has significantly higher energy demand than that of the adsorption methods [38] in order to remove micropollutants (Table 2).
Table 2: Literature data on the energy demand of ozonization [38].

Hydrogen Peroxide Treatment
In several cases H2O2 can be used to improve the formation of OH radicals [39]. The method is widely spread in the removal of organic pollutants. Rosenfeld et al. also reported that this process is suitable for the removal of pharmaceutical residues [40]. There is a tendency to supplement the hydrogen peroxide processes with UV treatment and other processes. Their effectiveness shows outstandingly good mineralization efficiency, which can be achieved in sevaral cases [41].
UV/H2O2 Combined Processes
Ultraviolet irradiation cleaves the oxygen-oxygen bonds at a suitable wavelength through a photochemical pathway, thus generating OH radicals from H2O2 (Eq. 1) [42]. During the wastewater treatment process, photon and OH radicals oxidize the micro-pollutants [43].

By absorbing a photon (eq.1), the quantum effect of OH inducement is 0.5 at 254 nm wavelength Figure 2, [44,45]. The formation of OH radicals in the UV/H2O2 system is pH and temperature independent [46], however, in practice the efficiency is influenced by the pH of the water since the OH radicals react with the CO3 2- and HCO3 - ions (Eqs. 2 and 3) [33] and other inorganic impurities dissolved in water [47].

According to Eqs. 2 and 3. the presence of hydrocarbonate and carbonate ions negatively influences the formation of OH radical and the mineralization of the organic materials [48], (Figure 2).
The removal efficiency of 23 different drugs was studied by H2O2/UVC oxidation procedure and the results are summarized in (Table 3).

Table 3: The removal efficiency of the UV/ H2O2 oxidation process for 23 micropolluting components at different H2O2 concentrations [Afonso-Olivares et al. 41].

Advanced Ulra-Violet Processes
Advanced UV-based oxidation processes have proven to be effective in removing organic micropollutants from waters. In case of heavily contaminated surface waters [47] and wastewaters outstanding results were obtained by this technology [49]. It is apparent from the work of Canonica et al. that the depletion of drug residues at neutral pH was for EE2 compounds was 0.4% for dicl8fenac, 26% for diclofenac and 15% for sulfamethoxazole.The elimination efficiency of 23 pharmaceuaticals are summarized in the following (Table 4), [41]:
*H2O2 dosage / Initial pollutant compound concentration ratio
Table 4: Elimination efficiency for 23 pharmaceuticals by advanced ultra-violet processes [Afonso-Olivares et al. 41].

Fenton Process
High-efficiency oxidation Fenton processes are now widely used in wastewater treatment, organic pollutant removal and other aspects of water protection. These are commonly used in industrial scale to remove non-biodegradable highly-stable materials [50] or for disinfection procedures [51,52]. The Fenton-based procedure is considered to be an efficient and commonly used process [53]. The first Fenton process was elaborated for the oxidation of maleic acid [54,32]. The Fenton process is the most effective at pH=3. The process consits of our steps: oxidation, neutralization, flocculation and sedimentation [55]. Basically, the removal of the organic materials takes place in two steps where the oxidation step and then coagulation occur at first [56]. During the oxidation of organic matter, OH radicals and coagulants are formed. This mechanism can be described according to the following equations (Eq. 4 to Eq 7):


Table 5 summarizes the advantages and disadvantages of the above-mentioned processes according to P.V. Nidheesh and R. Gandhimathi [32], (Table 5).
Table 5: Advantages and Disadvantages of Fenton Processes [Nidheesh and Gandhimathi, 32].

Catalytic Processes
In the course of water purification catalytic processes including heterogeneous, homogeneous catalytic as well as biocatalytic processes are also used. Homogeneous catalysis is an extremely selective process, usually transition metal complex or special organic compound is used with one or more reactants in one phase. Their disadvantage is the operational cost and the separation of the product [57]. The heterogeneous catalyst is immiscible with the reactants, and it forms a separate phase. The catalysts have high specific surface area and the reaction takes place in the pores. The advantage of this catalytic process that it easy to separate the catalysts from the product and catalysts are cheaper than the homogeneous catalysts. Their disadvantage is that in many cases the catalysts are not selective [58]. Biocatalysts are usually protein-type compounds with specific activity and catalyze only the predetermined reaction. Their advantage lies in their high selectivity. Their disadvantage is that they are extremely expensive and sensitive to temperature, pH, solvent, ionic strength, and product concentration [59].
A significant part of the papers dealing with AOP technologies focuses on heterogeneous photocatalysis. It is also apparent from the work of Ana R. Ribeiro et al. that 20% of coloring materials formed during the photocatalytic reaction [34]. Heterogeneous photocatalysis can be considered as a green technology for the removal of organic micropollutants. The most widely used catalyst is doubtless the TiO2. It shows high chemical and photochemical stability. The disadvantage of its use is the broadband energy demand (3.0-3.2 eV), which also covers the UV range in the electromagnetic spectrum [60]. Various combined processes have shown outstanding efficiencies in the removal of various micropollutants. Nuno et al. reported that photocatalytic procedures are the most effective in degradation and mineralization of organic micropollutants [61]. In Table 6, the results of several reaseach groups are summarized on photocatalytic treatments [61], (Table 6).
Table 6: Photocatalytic removal efficiency for drugs [Nuno et al., 61].

Electrochemical Oxidation
The electrochemical oxidation processes have been used in pilot plants in the past and in wastewater treatment projects [62]. In many cases the processes are used to reduce the detrimental discharges into surface water to avoid the ecological damage in the recepients [63]. In the processes hydroxyl radicals are formed directly by electrochemical reactions (anodic oxidation, AO) or with Fenton reagent. In the first case, the OH radicals are generated by the discharge at the anode, while in the latter case the Fenton reaction generates the OH radicals. Based on the experimental results the in situ hydroxyl radicals are the second strongest oxidizing agents according to our present knowledge [64]. Throughout the processes, the OH radicals can be produced in an environmentally friendly way, with a standard reduction potential of E = 2.8 V, which can be used to mineralize the micropollutants [65] during the non-selective oxidation. The processes are widely used to different sewage types. In many cases the processes have resulted in complete mineralization [66]. During the electrochemical oxidation, the OH radicals directly oxidize the pollutants due to the direct electron flow through the anode (Figure 3).
The anodic oxidation reaction is depicted in Figure 3. The reactions (a), (e), (f) show the active anodic reactions (a), (b), (c), (d). The reaction (a) results in the formation of hydroxyl radical M (OH); reaction (b) is the generation of metal oxide (MO), which results in reaction (c), the electrochemical transformation from the organic materials. Step (d) results in the formation of ozone (Michaud et al., 2003), and the formation of hydrogen peroxide can be observed. The electrochemical oxidation processes can also be efficiently used in the field of water purification. It was shown that after 7 hours of treatment, TOC removal of about 99% at 1000 mA current (16.2 mg/l solution) was achieved. It was also found that few intermediate degradation products were generated and effective mineralization was achieved [62]. According to Sirés and Brillas, the anodic electrochemical oxidation methods can be efficiently used for the removal of drug derivatives (Table 7), [67].

Table 7: Effectiveness of anodic oxidation for medicines Ignasi and Enric [67].

Ultrasonic Irradiation
Ultrasonic irradiation is considered a pollutant-free technology, and its use is widespread. It removes many micropollutants (ibuprofen, ethyl-paraben, paration, methyl-benzotrizole) [68]. The effectiveness of the treatment stems from the following: chemical effects, shock waves and shear stress [68]. Ultrasound spreads in the form of three-dimensional longitudinal waves. Wavefronts pass through the medium and cause pressure increase and decrease. Pressure fluctuation depends on the intensity of the sound waves. The pressure increase may be so high that it interrupts the continuity of the fluid medium by creating microscopic cavities. These rapidly generated cavities pulse through the liquid. As a result, 4000-6000 oC temperature and 300-500 bar pressure may occur locally in the treated wastewater. These extreme values result in the degradation of micropollutants [69].
Microwave Procedures
Microwave technology has gained ground in industrial use in addition to the well known household applications. During oxidation processes the oxidizing agents (potassium-persulfate) used under microwave irradiation will also be able to degrade some of the hardly degradable compounds (e.g.: perfluoro-octanoic acid, pesticides, azo-dyes). Yu-Chi and his colleagues demonstrated the defluorination and degradation efficiencies of compounds by the combination of microwave and oxidation treatment (Table 8) [70]
Table 8: Degradation efficiency of components by microwave treatment.

These micropollutants are efficiently broken down during the microwave process. High mineralization rate can be achieved in this way. It has been demonstrated that pH and temperature are important driving elements of this treatment [70]. Yu el al.studied the Fenton reaction combined with microwave treatment of pharmaceuticals of wastewaters. The efficiency varied between 40-60% depending on the Fenton dosage [71-94].
Discussion
Removal efficiencies of different pharmaceuticals from wastewaters are compared from the point of view of different technological processes used based on literature data. The combined ultraviolet and hydrogen-peroxide process, the ultraviolet process, the photocatalytic process, and the anodic oxidation process are evaluated for micropollutant removal. The removal of drugs (Paracetamol, 17-Beta-Estradiol, 17-Alpha- Ethinyl-estradiol, Trimethoprim, Sulfamethoxazole, Ranitidine, Propanolol, Paraxanthine, Omeprazol, Ofloxacin, Nicotine, Naproxen, Metronidazole, Metamizol, Ketoprofen, Ibuprofen, Gemfibrozil, Fluoxetine, Erythromycin, Diclofenac, Clofibrc acid, Ciprofloxacin, Carbamazepine, Caffeine, Bezafibrate, Atenolol) were studied by the above mentioned micropollutant removal technologies. The removal efficiencies for Ketoprofen, Ibuprofen, Erythromycin and Diclofenac from waters by different treatment technologies are illustrated in Figure 4 and 5. based on literature data since these drugs are widely used in our daily life. It can be concluded that the UV/H2O2 process and the photocatalytic process can be efficiently used for the removal of micropollutants from waters. For the selection of a fourth stage waste water treatment not only the micropollutant removal efficienies, but the financial aspects including the investment and operational costs must be considered as well (Figure 4 and 5).


Conclusion
From the point of view of the sustainable development it is an urgent task to deal with the fourth stage of wastewater treatment since the conventional wastewater treatment facilities cannot cope with the efficient removal of the micropollutants from wastewaters. Therefore it is a challange for the scientists to deal with new solutions to remove drug, pharmaceutical, pesticide residues from waters since the long-term human health impacts of these compounds cannot be clearly and fully understood. However, the preliminary studies on the health implications are alarming therefore it is a must to deal with these issues.
To read more about this article....Open access Journal of Chemistry and Biochemistry
Please follow the URL to access more information about this article
To know more about our Journals...Iris Publishers





No comments:
Post a Comment