Authored by Elaine Vanterpool*,
Abstract
Bacterial pathogens utilize strategic mechanisms to circumvent or evade host immune defenses. Opportunistic pathogens like Staphylococcus aureus, Serratia marcescens, and Klebsiella (Enterobacter) aerogenes, can cause local and systemic infections. Evasion of host immune defenses can also lead to sepsis. The complement system plays a critical role in killing pathogens and signaling other host immune processes to activate. Blocking or impeding the function of critical complement proteins like C3 can lead to bacterial evasion of host defenses and dissemination through the system. Previous studies show that the function of proteases produced by S. aureus and S. marcescens can be inhibited by synthesized silver nanoparticles (AgNP). This study evaluated the ability of S. aureus, S. marcescens, or K. aerogenes to degrade human C3 and if nanoparticles could block the potential degradation of C3 by bacterial proteases. It is hypothesized that degradation of C3 by S. aureus, S. marcescens, and K. aerogenes could be prevented by AgNPs. To evaluate the hypothesis, bacteria were incubated with purified C3 in the presence and absence of AgNPs, followed by analysis through immunoblot analysis. Results show that S. aureus and S. marcescens degrade human C3, but K. aerogenes did not. Further analysis demonstrates that AgNP protects the integrity of C3 from degradation by S. aureus and partially protects C3 from S. marcescens degradation. Data from this study supports the hypothesis.
Introduction
Staphylococcus aureus is a Gram-positive bacterium commonly found as part of the normal flora but can be an opportunistic pathogen leading to nosocomial and community-acquired infections [1]. The emergence of multidrug resistant S. aureus called Methicillin- resistant Staphylococcus aureus (MRSA) is a concern for the medical and scientific communities. S. aureus produces hydrolytic enzymes and other virulence factors which degrade host tissues and evade host immune defense strategies [2-5]. Serratia marcescens is a Gram-negative opportunistic pathogen implicated in infections of immunocompromised individuals and is a leading cause of infections in burn victims [6,7]. S. marcescens is an organism found in the respiratory and urinary tracts of hospitalized individuals and in the gastrointestinal tract of children. This pathogen produces virulence factors which too can cause destruction to host and evasion of the immune defenses [8-11]. Klebsiella (Enterobacter) aerogenes is a Gram-negative opportunistic pathogen associated with respiratory, urinary, and gastrointestinal infections [12]. All three opportunistic pathogens can cause very serious infections and complications in susceptible individuals.
Host immune defenses are critical for preventing microbial infections or dissemination of pathogens through the body. Some bacterial pathogens can destroy host tissues and disrupt normal homeostatic and immune functions. The innate defense, including complement system, can become activated by various triggers including bacterial pathogens [13-15]. The complement components are proteins that may be subjected to bacterial degradation as a strategy of evading host defenses. If proper immune defenses are disrupted, pathogens can disseminate throughout the body leading to more serious infections and complications [13-15]. Previous studies show that silver nanoparticles can inhibit the protease activities of S. aureus and S. marcescens [16]. This study further examined if silver nanoparticles could protect complement C3 from degradation by S. aureus, S. marcescens, or K. aerogenes. Complement C3 protein is an important, critical component of the innate immune response which aids in phagocytosis of pathogens, opsonization, and complement cascade activation for clearance of pathogens. Upon activation, C3 is cleaved into C3a (anaphylatoxin) and C3b. This will then result in C5 cleavage leading to a powerful immune response [13-15]. In this study, the immunoprotective effect of nanoparticles on preventing C3 degradation was evaluated. Protecting C3 from degradation may help maintain proper immune defense against serious pathogens.
Material and Methods
Growth and preparation of bacteria
Bacteria S. aureus, S. marcescens and K. aerogenes were grown to stationary phase (optical density 600 (OD600) of values over 1.0) in brain heart infusion broth (BHI) at 37° C for 20 hours. Bacterial cells were harvested by centrifugation and resuspended in IX Phosphate Buffered Saline (PBS) to an OD600 of 0.6.
Synthesis of Silver Nanoparticles (AgNP)
Silver nanoparticles were synthesized similarly as previously described [16,17]. Briefly, silver nanoparticles (AgNP) were synthesized as follows. 0.01M AgNO3 was placed in a round bottom flask and heated. 1% trisodium citrate was added and continued heating with stirring until the solution was an amber yellow color. The sample was removed from the heat immediately with continued stirring until it cooled to room temperature. A sample of the solution was evaluated in the UV-vis spec to confirm identity. Characteristic absorbance at 410nm was recorded and particles had an average diameter of 32nm as determined by Dynamic Light Scattering (DLS) spectrophotometry [16,17].
Treatment of Complement C3 protein with pathogens
Evaluation of bacterial impact on C3: To determine if pathogens can degrade complement C3 protein, 100μl of resuspended S. aureus, S. marcescens or K. aerogenes grown to stationary phase were incubated with 100 ng of commercially available C3 protein (TexGene) for a course of 6 hours at 37°C. After incubation, samples were centrifuged, and cell-free supernatants containing the C3 proteins were analyzed by immunoblot (Western blot) analysis. The negative control in this study was C3 incubated with 100μl of PBS and no bacteria.
Evaluation of AgNP protection of C3 integrity: To evaluate if AgNP can protect C3 from bacterial degradation, 100μl of suspended bacteria (S. aureus, S. marcescens and K. aerogenes) were incubated with 100ng of C3 (TexGene) in the presence of 50 μl of silver nanoparticles for 6 hours at 37°C. Bacterial cells were removed via centrifugation and the cell-free fraction were separated by SDSPAGE and then cleavage or degradation was evaluated by Western blot analysis. Controls for this study includes C3 incubated with Ag- NPs, and C3 incubated with PBS (no bacteria).
Western Blot Analysis: Experimental samples were prepared for SDS-PAGE and Western blot as follows: 65 μl of experimental sample, 10 μl of reducing agent (Life Technologies), 25 μl of LDS sample buffer (Life Technologies) then heated to 80oC for 10 minutes to denature samples. The sample proteins were separated using standard SDS-PAGE technique using a gradient 4-12% Bis-Tris gel (Life Technologies). After electrophoresis, the proteins were then transferred to PVDF membrane (Life Technologies) and C3 protein was probed using primary antibodies rabbit anti-C3 antibodies (TexGene). The Western Breeze Chromogenic Western blot kit (Life Technologies) was used to detect the integrity of the C3 protein. Instructions were followed according to manufacturer’s recommendations (Life Technologies).
Results and Discussion
Bacterial degradation of complement C3
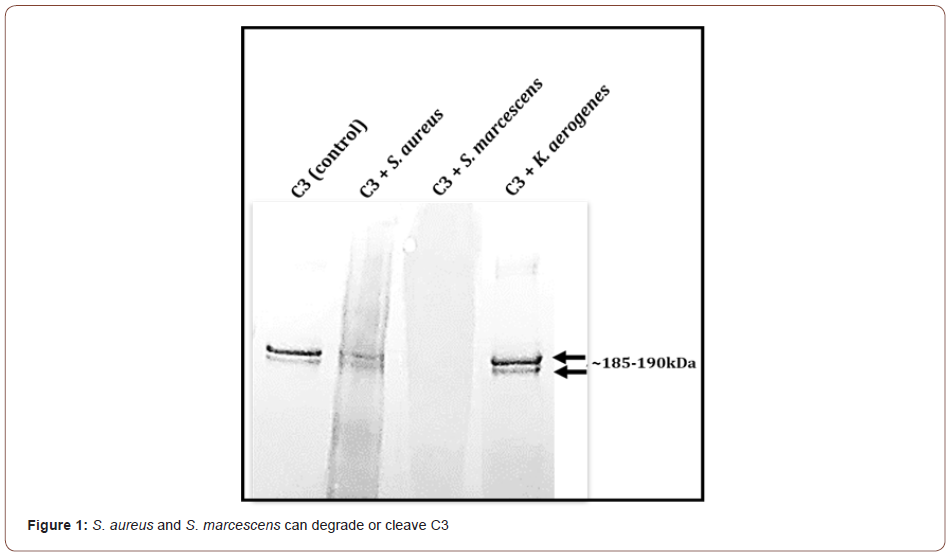
To evaluate if S. aureus, S. marcescens, and K. aerogenes could degrade C3, immunoblot (Western blot) analysis of C3 incubated with the three bacteria was done. The integrity of the intact 185- 190kDa C3 bands was evaluated. Results show that in comparison to the untreated C3 control, S. marcescens completely degrades the complement C3 protein resulting in a smear with no observable bands around the185-190 kDa (Figure 1). S. aureus incubation with C3 results in a reduction in the 185-190 kDa bands intensity compared to the untreated negative control (Figure 1). K. aerogenes did not appear to degrade C3 (Figure 1). These findings point to a possible strategy that is used by certain pathogens to escape immune defense. Normally one of the three complement pathways (classical, lectin, or alternative) are activated by a particular stimulus. No matter which complement pathway is triggered, they all converge at complement C3. Once activated, C5 becomes cleaved and activated which leads to the formation of the membrane attack complex. If complement C3 is degraded, the pathogen may escape elimination by the complement cascade which can lead to disease or damage by the pathogen. It would be beneficial for the host to preserve C3 integrity and function.
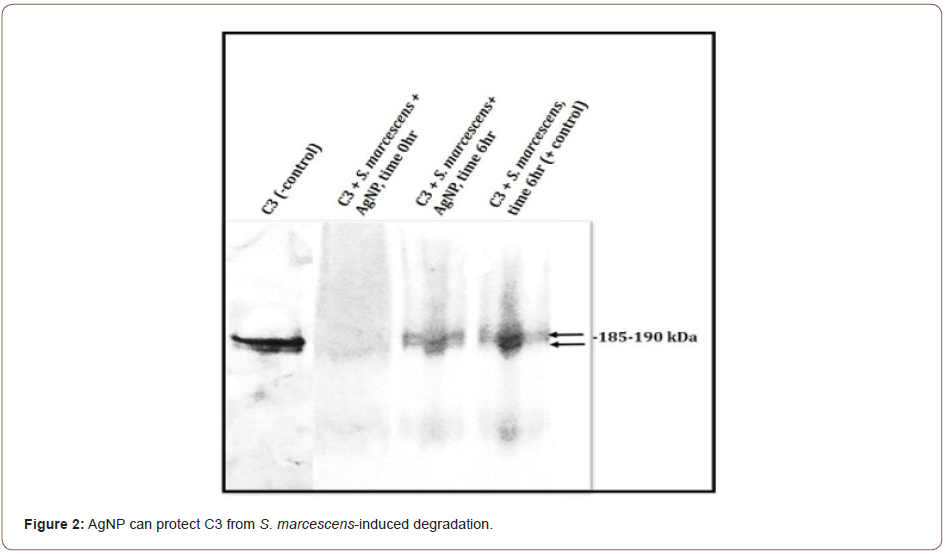
AgNP partially protects C3 from S. marcescens
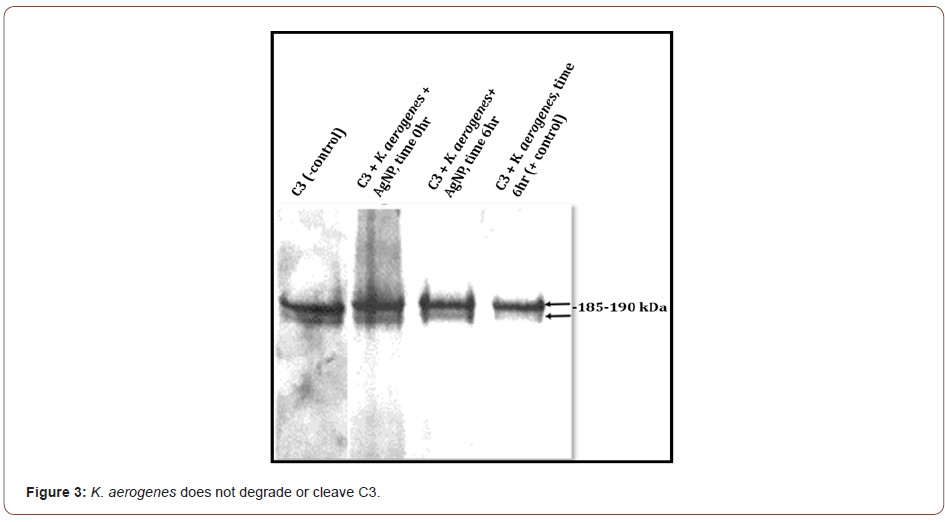
C3 incubated with S. marcescens results in the loss of the C3 protein band integrity by the presence of visible smearing and complete degradation of C3 (Figure 2). C3 incubated with S. marcescens in the presence of AgNP shows some intact 185-190kDa C3 complement protein bands. Degradation is not completely prevented, but some C3 is preserved (Figure 2). In contrast, K. aerogenes did not result in any C3 cleavage in the presence or absence of AgNP (Figure 3). This data suggest that AgNP can provide some protection of C3 from S. marcescens. Our lab is currently investigating if bimetallic gold-silver nanoparticles (AgAuNP) could provide better protection of C3. Previous studies from our lab show that AgAuNP can reduce the proteolytic activities of S. marcescens more than monometallic AgNP. If there is greater inhibition of proteolytic function, there may be less degradation of C3, preserving part of the host innate immune defense.
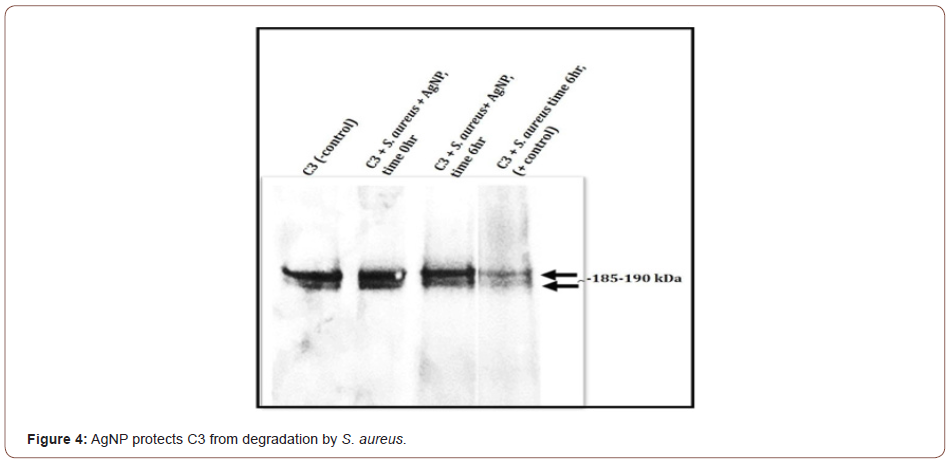
AgNP protects C3 from S. aureus-induced degradation or cleavage
Results with S. aureus demonstrate that C3 is partially degraded or cleaved by S. aureus (Figure 4). After incubating C3 with S. aureus in the presence of AgNP, no visible detection of C3 degradation is observed in comparison to the untreated C3 control. The 185-190 kDa C3 bands appear to be preserved (Figure 4). Of special note is that without the presence of AgNP, S. aureus not only partially degrades or cleaves C3, but an immunoreactive band approximately 49kDa is also observed that is absent when AgNP is present (data not shown). Studies in our lab are ongoing to investigate if C3 from total serum would be protected by AgNPs. These studies are currently evaluating C5 integrity, cleavage of C5 into C5a and C5b, and the downstream mechanisms involving the formation of the membrane attack complex and certain inflammatory reactions. We are also investigating the components of the specific components of the classical, alternative, or lectin pathways. Interestingly, other research has shown that Staphylococcus pseudintermedius was able to evade the host immune response by altering the function of complement and antibodies [18].
Conclusion
Complement is a critical component of host innate immunity. Disruption in key complement components can have dire consequences including preventing the clearance of bacterial pathogens possibly leading to sepsis, and septic shock. Our findings show that AgNP can protect C3 from degradation and/or cleavage by S. aureus and S. marcescens. These mechanisms can also be investigated in other important human pathogens. In addition, the findings of this study can be used by the medical and scientific communities to identify strategies in combating complications caused by bacterial pathogens.
To read more about this article...Open access Journal of Biology & Life Sciences
Please follow the URL to access more information about this article
To know more about our Journals...Iris Publishers





No comments:
Post a Comment