Authored by Singh Yuvaraj*,
Introduction
Acute pancreatitis (AP) is an important cause of hospitalization amongst gastrointestinal disorders in the United States. The diagnosis of the acute presentation is clinical, but the major challenge is predicting the disease course and outcome. This is critical to determine where the patient may be monitored [1- 3]. Acute pancreatitis (AP) is an inflammatory disease of the pancreas that affects the pancreatic parenchyma. It may range from a mild self-limiting form, i.e., acute interstitial pancreatitis, to a more rapidly fatal condition, i.e., acute necrotizing pancreatitis. The inflammation may extend beyond pancreatic tissue to the peripancreatic region and is usually associated with a systemic inflammatory response. If left untreated, it may end up causing multi-organ dysfunction syndrome [4].
Common etiologies of acute pancreatitis are listed below [3-6].
Alcohol use disorder
Gallstones
Hypertriglyceridemia
Drug-induced pancreatitis
Post-procedural(endoscopicretrograde cholangiopancreatography or abdominal surgery)
Ampullary stenosis, formerly known as sphincter of Oddi dysfunction type I
Autoimmune pancreatitis, type I (systemic IgG4 diseaserelated), and type II
Viral infection (coxsackie, cytomegalovirus, echovirus, Epstein- Barr virus, hepatitis A/B/C, human immunodeficiency virus {HIV}, mumps, rubella, varicella)
Bacterial infections (campylobacter, legionella, leptospirosis, mycobacteria, and mycoplasma)
Trauma
Smoking
Congenital anomalies (annular pancreas, pancreatic divisum) Genetic disorders (hereditary pancreatitis, cystic fibrosis, alpha 1-antitrypsin deficiency)
Hypercalcemia
Parasitic infections (Ascaris lumbricoides, Cryptosporidium, Clonorchis sinensis, Microsporidia)
Renal disease (Hemodialysis)
Toxins (Scorpion bites, organophosphate poisoning)
Vasculitis (Polyarteritis nodosa, Systemic lupus erythematosus)
Gallstone pancreatitis is the most usual type, which is caused by duct obstruction by gallstone migration. Obstruction of duct promotes pancreatitis by increasing duct pressure and subsequent unregulated activation of digestive enzymes [7-8]. Alcohol-induced pancreatitis is the second most common cause. In about 8% of cases of AP related to alcohol, mutations in the pancreatic secretory trypsin inhibitor gene (SPINK1) are seen [9]. Another cause of AP is hypertriglyceridemia and is estimated to make up 1%-4% of cases [9-11]. It is due to the hydrolysis of excessive triglyceriderich lipoproteins causing high concentrations of free fatty acids, leading to injury to the vascular endothelium and acinar cells of the pancreas [12]. Infectious causes of AP have been investigated widely for a long time. Parasites like Echinococcus, Plasmodium, Fasciola, Ascaris, Toxoplasmosis, and Cryptosporidium have been documented to be causes of AP as well [13]. About 15%-20% of patients with AP have a probability of developing severe disease and have an extended hospital stay with complications including death [13-16]. Several theories have been postulated to understand better and describe the mechanism by which AP occurs but are still inconclusive [17].
Clinical Presentation of Acute Pancreatitis
The cardinal symptom of acute pancreatitis is acute onset of persistent upper abdominal pain, usually with nausea and vomiting. The usual locations of the pain are the epigastric and periumbilical regions. The pain may radiate to the back, chest, flanks, and lower abdomen. Patients are usually restless and bend forward (the kneechest position) to relieve the pain because the supine position may exacerbate the intensity of symptoms [18]. Physical examination findings are variable but may include fever, hypotension, guarding/ rigidity on abdominal exam, respiratory distress, and abdominal distention [19].
Diagnosis of Acute Pancreatitis
The classical teaching is that a serum amylase level that is three to four times greater than the upper limit of normal along with typical signs and symptoms is diagnostic of acute pancreatitis. The biochemical measurement of trypsinogen activation peptide and trypsinogen-2 is more useful as a diagnostic marker due to their accuracy, but their use is limited by availability [20]. Lipase has higher diagnostic accuracy compared to amylase as the serum lipase levels are elevated for a more extended period [21]. During an attack of acute pancreatitis, the elevation of alanine aminotransferase to >150 IU/L is predictive for a biliary cause of acute pancreatitis [22]. A previous meta-analysis has indicated that this threefold elevation in alanine aminotransferase has a positive predictive value of 95% in diagnosing acute gallstone pancreatitis [23]. Early elevated levels of urinary trypsinogen activation peptides are associated with severe acute pancreatitis [24].
Review
Overview of Parasitic Causes of AP
Ascaris lumbricoides is the most common parasite implicated in AP, as mentioned by Parenti, et al. [19] in their extensive review. The mechanism of pancreatitis is due to the physical obstruction of the pancreatic duct due to the adult worm. It is more common in children due to the smaller size of their pancreatic-biliary tree [19]. In a prospective study carried out in India; ascariasis was the leading parasitic cause of pancreatitis in 59 of 256 patients (23%) compared to 112 patients (44%) with gallstone pancreatitis [20]. The Chinese liver fluke, Clonorchis sinensis, was also reported in some cases of AP due to the obstruction of the pancreatic duct [21]. Plasmodium falciparum, common protozoa implicated in malaria, has several case reports where patients with malaria complicated by multi-organ dysfunction syndrome had AP [22].
Although infrequently associated with AP, taeniasis has been reported as a possible helminthic cause of AP in a case published in 2005. In this instance, the patient presented with typical symptoms of AP and markedly elevated serum amylase and lipase, including a history of the passage of white worms with the stool for four years. The proglottids were extracted from the duodenal mucosa with ultrasound findings of a dilated common bile and pancreatic duct, which provided possible evidence of a tapeworm passing through the biliary tree. The patient responded to anti-helminthic drugs with clinical improvement [23] Other parasites implicated in AP include Opisthorchis species [24], Fasciola hepatica [25- 26], and Echinococcus granulous which may cause AP due to the compression of the pancreatic duct by the cyst [19]. The summary of parasitic infectious etiology of acute pancreatitis has been depicted in (Table 1).
Table 1: Summary of the parasitic infectious etiology of acute pancreatitis.
Case Series on Parasitic Causes of AP
Ascaris Lumbricoides
Ascaris lumbricoides is one of the most common helminthic infections worldwide. With an overall prevalence of 25%, an estimated 1.4 billion people are infected, and 1.2 to 2 million cases of the clinical disease occur per year, spanning 20,000 deaths. This is particularly prevalent in areas with poor sanitation and humid climates of tropical regions. Children between the ages of 2 to 10 years are most commonly affected, and prevalence decreases after 15 years. The life cycle starts after ingesting the eggs from contaminated food, soil, vegetables, and water. After hatching, larvae emerge in the duodenum and then migrate through the portal, and systemic circulation reaches the liver. They migrate to the alveoli and ascend through the bronchial tree and throat. Here they are swallowed and mature into adult worms [27] As stated by Klimovskij, et al. [28], “The roundworms are actively motile, have wandering nature, and can migrate from their natural habitat in the duodenum and proximal jejunum into the ampulla of Vater entering the bile or pancreatic duct to cause cholangitis or pancreatitis.”
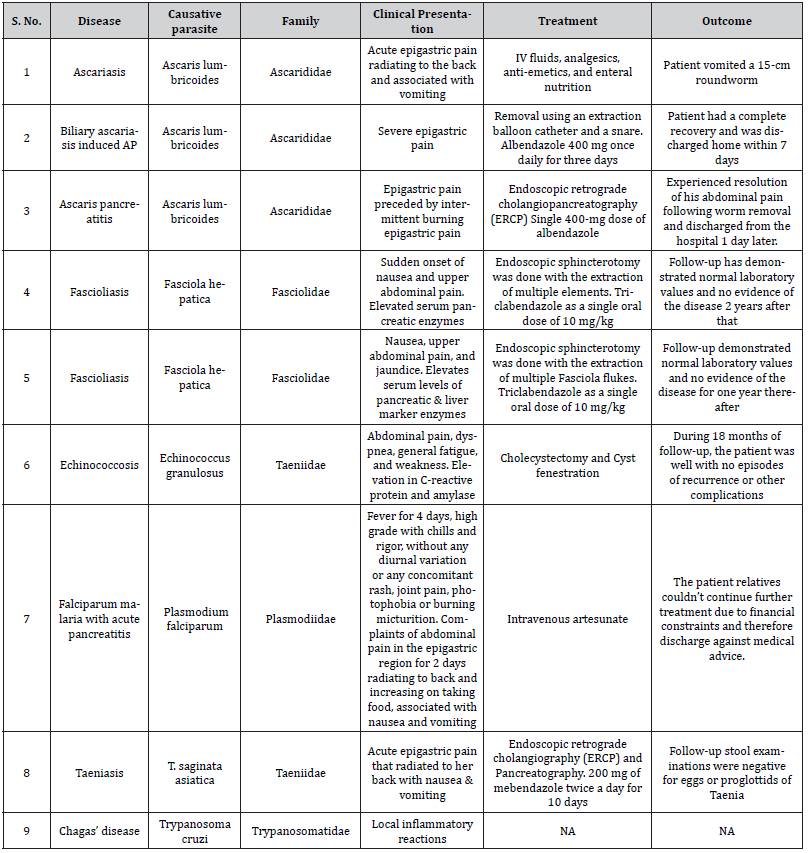
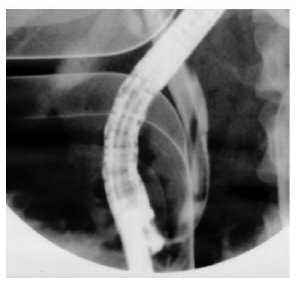
Hussain, et al. [29] reported a case of AP due to ascariasis wherein a twenty-five-year-old male patient presented with a chief complaint of acute epigastric pain radiating to the back associated with vomiting. Initial lab investigations revealed increased serum amylase and lipase. The computed tomography (CT) scan revealed edematous pancreas and significant peri-pancreatic fat stranding (Figure 1). He was managed symptomatically with intravenous fluids, analgesics, anti-emetics, and enteral nutrition. However, the cause remained undetermined as the authors ruled out the possible etiologies of AP until one day, the patient vomited a 15- cm roundworm (Figure 2). After that, his condition improved dramatically. Thus Hussain et al. highlighted ascariasis as a possible etiology of AP in regions where it is geographically endemic.
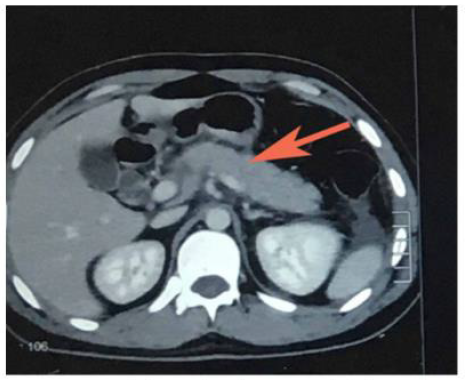

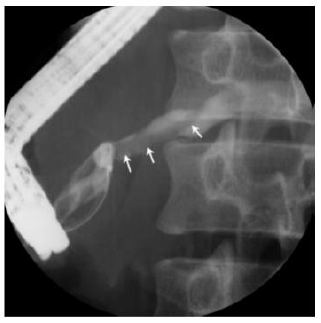
Phisalprapa, et al. [30] reported a case of a 33-year-old woman living in urban Thailand who presented with severe epigastric pain, later diagnosed with AP secondary to Ascaris lumbricoides. Her preliminary investigations showed no eosinophilia or Ascaris eggs in stool examination; however, her abdominal CT image showed common bile duct dilatation. The parasite was found when a diagnostic endoscopic retrograde cholangiopancreatography (ERCP) was performed (Figure 3). The cholangiography revealed a roundworm in the common bile duct which was successfully removed using an extraction balloon catheter and a snare. Microbiological examination of the parasite revealed a 22 cm long adult form of Ascaris lumbricoides. Through the findings of this study, the authors delineated that Ascaris lumbricoides is an uncommon cause of biliary obstruction with complications. Endoscopic removal is the treatment of choice in addition to antihelminthic medications.
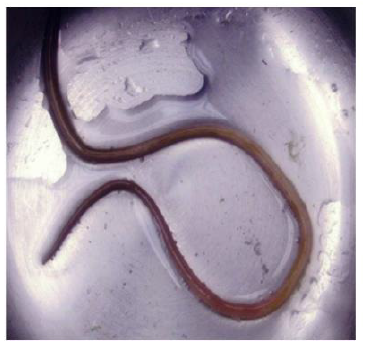
Carter, et al. [31] reported a case of Ascaris pancreatitis in an 18-year-old Hispanic man who presented with a 2-week history of continuous moderate to severe epigastric pain that was preceded by six months of intermittent burning epigastric pain worsened with eating. Prior episodes typically lasted 1-2 days and then resolved spontaneously. A complete blood cell count demonstrated mild leukocytosis (12.3 X 109/L), elevated amylase (539 U/L), and lipase (4671 U/L) levels. The patient’s abdominal CT image reportedly demonstrated a dilated pancreatic duct. The magnetic resonance cholangiopancreatography (MRCP) showed mild peripancreatic edema and a diffusely dilated pancreatic duct containing a smooth linear filling defect. Biliary ducts were not dilated, and there was no evidence of an ampullary mass. ERCP was performed for further evaluation. At initial inspection, the major papilla appeared dilated and contained a tubular, mucus-appearing foreign body, which was extracted in its entirety with biopsy forceps measuring 10.5 cm in length (Figure 4). The pancreatic duct was then cannulated, and contrast material was injected. Fluoroscopic images from ERCP demonstrated a diffusely dilated main pancreatic duct containing a second tubular filling defect (Figure 4). After guidewire placement and balloon extraction, a second vermiform structure measuring 14 cm was also removed [31].
Fasciola Hepatica
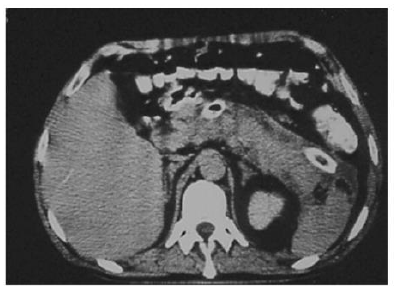
Fascioliasis (liver fluke disease) is a disease of sheep, cattle, and other herbivorous animals observed virtually throughout the world. Humans are only accidental hosts for Fasciola hepatica. F. hepatica is a zoonotic liver fluke that can cause disease in humans. F. hepatica is known to cause bile duct inflammation and obstruction but is rarely reported to cause acute pancreatitis. ERCP shows distinct features in some patients with fascioliasis, but the condition might be overlooked in chronic cases. Parasite removal during ERCP is one therapeutic option in patients with acute obstructive biliary tree disease due to Fasciola hepatica. Echenique-Elizondo, et al. [32] reported a case of A 31-year-old female experiencing a sudden onset of nausea and upper abdominal pain. Abdominal ultrasonography (USG) and CT scan showed diffuse enlargement of the pancreas (Figure 5). A cholangiogram depicted dilatation and numerous filling defects images in the main bile duct (Figure 6). An endoscopic sphincterotomy was done to extract multiple elements of the parasite (Figure 7). Treatment with triclabendazole as a single oral dose of 10 mg/kg was initiated. Follow-up demonstrated normal laboratory values and no evidence of the disease two years later. Through the study, the authors demonstrated that fascioliasis must be considered on the differential diagnosis of abdominal pain, especially if associated with eosinophilia. Pancreatitis is an infrequent complication seen secondary to biliary involvement.
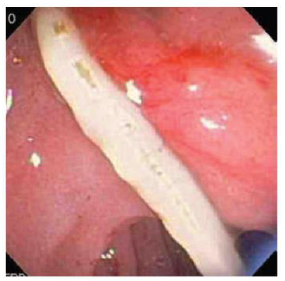
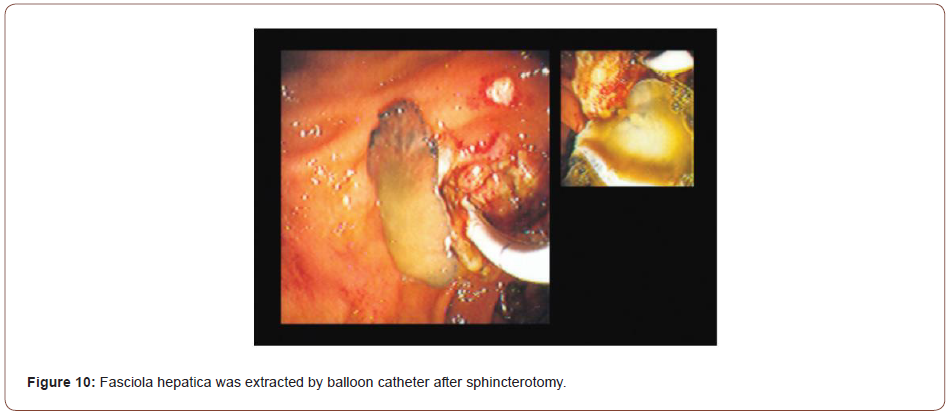
Sezgin, et al. [25] reported a case of a 51-year-old female admitted with complaints of sudden onset of nausea and upper abdominal pain. She cited no history of drug abuse or alcohol ingestion, gallstone disease, abdominal trauma, or surgery. Physical examination revealed severe tenderness in the epigastrium with hypoactive bowel sound and fever (38°C). Laboratory data on admission showed elevated serum amylase (1046 IU/L) and lipase (1538 IU/L). White blood cell count was 7,750 mm-3, and eosinophil count was 9.7%. Alkaline phosphatase (ALP) 148 IU/L, bilirubin 1.3 mg/dl, alanine aminotransferase (ALT) 325 IU/L, aspartate aminotransferase (AST) 612 IU/L, and lactate dehydrogenase (LDH) 904 IU/L; serum calcium was within reference ranges. Abdominal USG showed bile duct dilation and hyperechoic material filling the common bile duct and diffuse enlargement of the pancreas. A cholangiogram depicted dilation and numerous motile curvilinear filling defect images in the common bile duct and irregularities at the bile duct wall. An endoscopic sphincterotomy was done with the extraction of multiple Fasciola hepatica flukes (Figure 8).
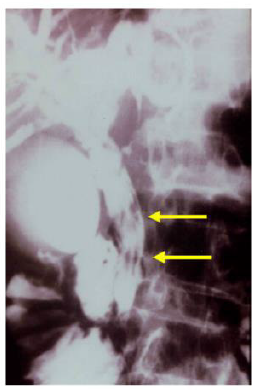
Sezgin et al. reported another case of a 70-year-old male admitted with complaints of nausea, upper abdominal pain, and jaundice for three days later diagnosed with AP secondary to multiple liver flukes infestation of the pancreatic duct [25]. Summarily, case studies of Fasciola hepatica revealed that fascioliasis must be considered in the differential diagnosis of abdominal pain, especially if associated with eosinophilia. Pancreatitis is an extremely rare complication that can be associated with biliary involvement. A high index of suspicion and specific USG findings are helpful in the diagnosis. Serological studies and ERCP can confirm the diagnosis.
Echincoccosis Granulosus
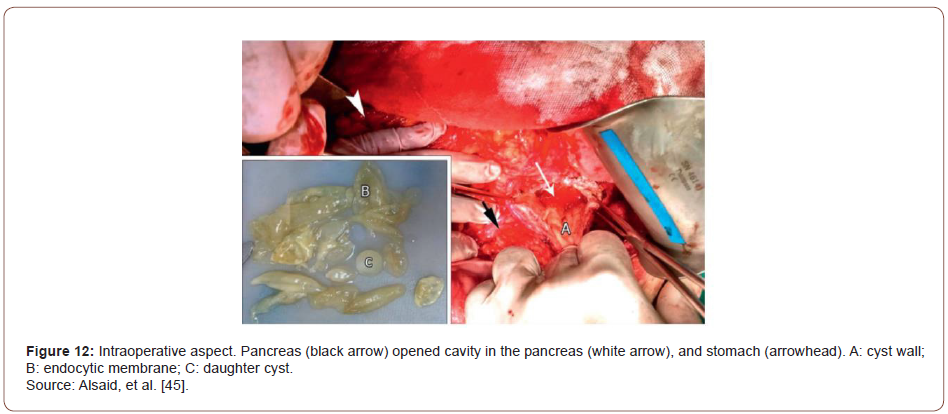
Also known as hydatid disease, or hydatidosis, are alternative names of the zoonotic parasitic diseases caused by the tapeworm Echinococcus granulosus. It is endemic in Mediterranean countries, the Middle East, South America, and the Indian subcontinent [33-36]. Four types of Echinococcus lead to infection in humans; Echinococcus granulosus is the most common parasite causing cystic echinococcosis with a larval stage that represents more than 95% of cases [37]. It can infest various organs, and the invasion of the liver and lungs accounts for 90% of cases [38]. Other involved sites are the muscles, bones, kidneys, brain, spleen, and pancreas. Pancreatic localization is a rare situation of hydatidosis, representing 0.2% of cases [39]. Alsaid, et al. [39] reported a case of pancreatic hydatid cyst causing AP in a 34-year-old man admitted with diffuse abdominal pain, dyspnea, and weakness. His abdominal USG revealed a 5 cm heterogeneous area in the body of the pancreas with peripancreatic fluid, gallstones with a thick gallbladder wall, and multiple cysts in the left kidney. Abdominal CT showed a heterogeneous collection of fluid with a thick wall of 12 × 4 cm in size along the body of the pancreas and left colic angle (often an abscess or a pseudocyst) with infiltration of adipose tissue around it and mild thickness at the wall of the colon. Laboratory investigations were within normal levels except for an elevation in C-reactive protein (18.1 mg/dl) and amylase (765 U/L). Hence, the patient was diagnosed with acute pancreatitis. A month later, the CT scan of the chest and abdomen was similar to the previous finding, and the pancreatic cyst measuring 13.5 × 7 cm stretched down through the peritoneal cavity in front of the mesenteric vessels. Laboratory values were normal, a primary diagnosis of pancreatic pseudocyst was probable, and surgery was planned.
Intraoperatively, there was edema in the pylorus, transverse mesocolon, the head and body of the pancreas, and hepatoduodenal ligament. After entering the lesser sac, a large mass 35 × 20 ×15 cm in size was found between the tail of the pancreas, spleen, left colic angle, left kidney, stomach, and diaphragm. The cyst was hard to dissect from the neighboring structures. Clear liquid was aspirated, proposing the probability of a hydatid cyst. On cyst fenestration, multiple daughter cysts were evacuated; the endocyst membrane was removed (Figure 9). A Foley catheter was placed in the residual cavity. The final diagnosis was pancreatic hydatid cyst. Through the experience of this rare case of pancreatic hydatid cyst causing AP, Alsaid, et al. [39] stated that despite pancreatic hydatid cyst being rare, it could be considered an etiology when common causes of AP are ruled out. Hydatid cysts should be counted as a significant differential diagnosis for cystic lesions of the pancreas and other organs, especially in endemic regions.

Plasmodium Falciparum
Malaria is one of the most common parasitic infections in humans, with a high incidence rate in India. 1.8 % of cumulative deaths before the age of 70 years are attributed to malaria [40]. Severe malaria can present with a wide spectrum of complications, including acute kidney injury, liver injury, cerebral involvement, coagulopathy, and anemia. Acute pancreatitis secondary to malaria is a very rare complication [41]. Roy, et al. [42] reported a case of AP due to falciparum malaria in a 45-year-old male. The case was presented with a history of high-grade fever of 4 days with chills and rigors, without any diurnal variation or any concomitant rash, joint pain, photophobia, or burning micturition. He also complained of abdominal pain in the epigastric region for two days radiating to the back and increasing on taking food, associated with nausea and vomiting. These symptoms were followed by confusion and decreased urine output. On examination, the patient was febrile with a Glasgow coma scale (GCS) of E3V2M5 (10 out of 15). Abdominal examination revealed tenderness in the epigastric region without guarding, rigidity, or organomegaly. The rest of the systemic examination was unremarkable. He was kept nil per oral (NPO), and a nasogastric tube was inserted. A bedside ultrasound abdomen was performed, revealing mild hepatomegaly, obscured pancreas, and no gall bladder stones. He was started with intravenous fluid (0.9 % normal saline), empirical antimicrobials (ceftriaxone), and opioid analgesics. Subsequent blood investigations revealed a deranged renal and hepatic function test consisting of raised lipase (1047 U/L) and amylase (533 U/L) levels.
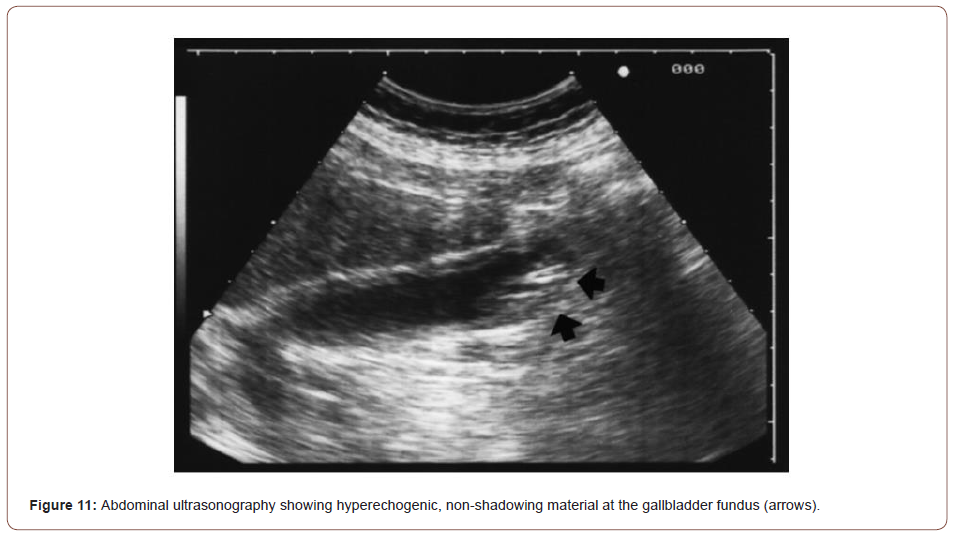
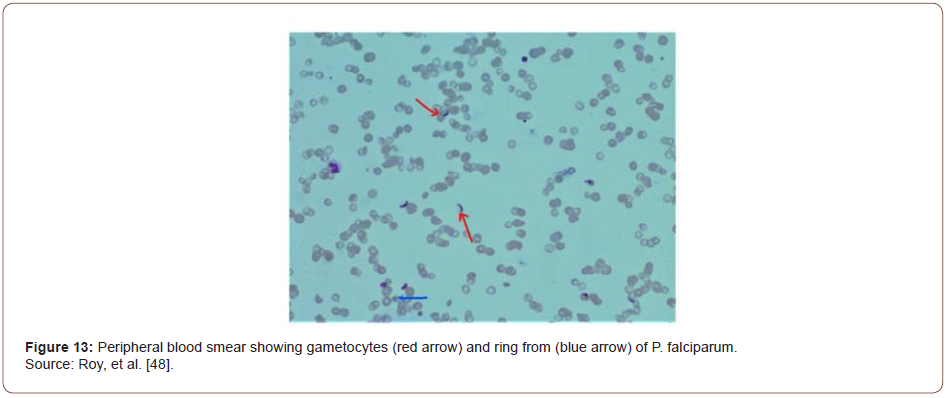
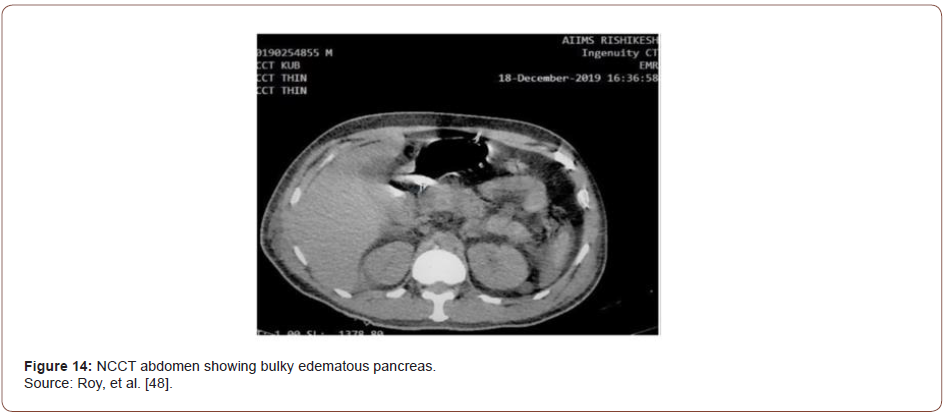
Based on characteristic pain and elevated pancreatic enzymes, the patient was diagnosed with acute pancreatitis as per Atlanta criteria [43]. His acute febrile illness work-up turned positive for Plasmodium falciparum. In peripheral smear, multiple gametocytes and ring forms of P. falciparum were detected (Figure 10). He was started on intravenous artesunate as per standard guidelines. CT of the abdomen was performed where a bulky, edematous pancreas with few dilated bowel loops was noted (Figures 11-15). Hence a diagnosis of P. falciparum malaria with acute pancreatitis was made.
Taeniasis
Taeniasis is highly prevalent among the aborigines in Taiwan. Overall infection of taeniasis is 11% among Taiwanese aborigines [44]. They acquire infection Taenia by eating the meat or liver of pigs. Evidence suggested that the Asian Taenia is a subspecies of T. saginata and it has been renamed T. saginata asiatica [45]. The cysticerci of T. saginata are recovered mainly in the liver of pigs, which are the intermediate hosts. Most people with Taeniasis are asymptomatic and only become aware of the infection when they pass proglottids in their stools. However, some complain of pruritus ani (77%), nausea (46%), abdominal pain (43%), dizziness (42%), increased appetite (30%), and other mild gastrointestinal symptoms [46].
Fan, et al. [46] reported the case of a 52-year-old Taiwanese aboriginal woman with acute pancreatitis, a rare but potentially serious complication of tapeworm - T. saginata infection. The patient developed acute epigastric pain that radiated to her back. She also had nausea and vomiting; the vomitus included fragments of a strobila. She presented for evaluation after the pain had persisted for two days. She had noticed long white worms in her stools for four years, but she ignored the finding since Taeniasis is not uncommon on Lanyu Island. On examination, the patient was of medium build and did not appear malnourished or jaundiced. She had mild direct epigastric tenderness but no rebound tenderness or muscle guarding. There was no right upper quadrant tenderness. She had a white blood cell count of 11,000 /mm3, a serum amylase level of 3,045 U/L, a serum lipase level of 2,464 U/L, a serum aspartate aminotransferase (AST) level of 2,678 U/L, a serum alanine aminotransferase (ALT) level of 1,466 U/L, and a total bilirubin level of 0.9 mg/dL.
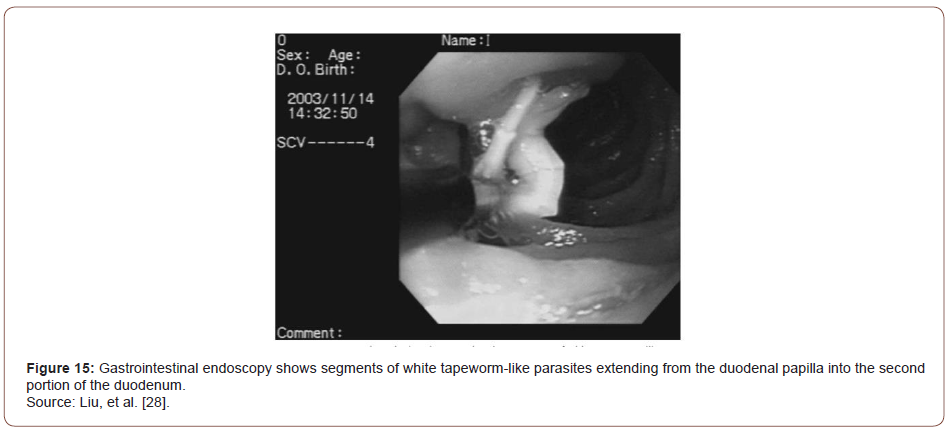
Abdominal sonography on the day of admission showed dilatation of the common bile duct and pancreatic duct and linear hyperechoic material in the gallbladder sac with an acoustic shadow. Cholelithiasis was the most likely diagnosis, although the possibility of worms in the gallbladder could not be excluded. Gastrointestinal endoscopy showed long strobilae of tapeworms in the duodenum involving the papilla. Two strobilae of tapeworm-like parasites measuring up to 15 cm in length and 2 mm in diameter within the papilla were extracted with forceps. Endoscopic retrograde cholangiography (ERCP) performed three days after admission showed mild dilatation of the common bile duct but no worms in the biliary tract. Pancreatography was not performed at that time because the patient did not tolerate the endoscopy well. Treatment with 200 mg of mebendazole twice a day was started on the first day of admission and continued for 10 days. Three days after admission, dead proglottids of the tapeworm were still seen in the stool, but the patient’s symptoms had resolved. Her AST and ALT levels decreased to 110 U/L and 331 U/L, respectively. At followup three weeks later her amylase level was 228 U/L and the lipase level was 716 U/L; the AST and ALT levels were within normal limits. In addition, follow-up stool examinations were negative for eggs or proglottids of Taenia. Through the experience of this case, the authors demonstrated that pancreatitis secondary to tapeworm infection can be treated after the removal of tapeworm by endoscopy followed by anthelmintic treatment.
Trypanosoma Cruzi
Chagas disease formally called American trypanosomiasis is one of the most important endemic tropical afflictions. It is found from the southern United States to Argentina; according to the World Health Organization, there are 16 to 18 million infected people in Central and South America [47] This disease is caused by the protozoan Trypanosoma cruzi, which is transmitted to man by hematophagus triatomine insects (Hemiptera: Reduviidae) when infective trypomastigotes forms are discharged with the vector’s feces during feeding. When the affected person scratches the site of the bite, infection occurs. The trypomastigotes form circulates in the blood, and after penetrating host cells, transform into amastigote form, giving rise to pseudocysts.
Chagas’ disease presents an acute course with fever, malaise, and nonspecific signs of infection. The clinical course is usually discreet and may be unnoticed. It is difficult to identify it in the acute phase because the signs and symptoms are those of a nonspecific infection, and patients normally do not seek medical help. In general, the only symptom of infection is a local inflammatory reaction that occurs at the site of infection in 80% of the cases [47]. In a research study conducted by Corbett, et al. [48] aimed to evaluate the involvement of the pancreas in acute experimental Chagas’ disease in a mouse model by histopathological characterization, ten BALBc mice were injected with about 100000 forms of the Y strain of Trypanosoma cruzi. After 14 days of infection, fragments of the pancreas were processed by conventional paraffin embedding and hematoxylin-eosin staining. Results revealed ruptured pseudocysts and release of parasites to the extracellular medium caused by necrosis of acinar and duct cells with foci of fat. These were the most striking histopathological features of acute Chagasic pancreatitis.
Clonorchis Sinensis
Clonorchiasis is a parasitic disease that is often found in Japan, Korea, China, Hong Kong, Taiwan, and countries in Southeast Asia [49-51]. The incidences of Clonorchiasis is around 38 million worldwide [52]. Clinical manifestations depend on the number of flukes in a patient, the period of infestation, and the complications. Patients with light infestation usually have neither signs nor symptoms. However, in patients with heavy infestation, the extrahepatic bile duct, the gallbladder, and the pancreas are involved, manifesting symptoms related to the involved organs. Literature reports show that Clonorchis sinensis infestation has been associated with pancreatitis [53].
An original research study conducted by Kim et al. described the CT characteristics in patients with Clonorchis sinensis pancreatitis. During a 6-year period, 1142 patients underwent CT of the abdomen for evaluation of symptoms related to the hepatobiliary pancreatic system. Of the 1142 patients. 92 (8.1%) were shown to have C. sinensis on the basis of operative findings, an analysis of stool, an intradermal test for Clonorchis, or any combination of the three. Of these 92 patients, seven (7.6%) were found to have C. sinensis pancreatitis. The diagnosis of C. sinensis pancreatitis was made in four patients on the basis of operative findings and in the remaining three patients on the basis of clinical findings such as abdominal pain and raised pancreatic enzymes in blood or urine [54].
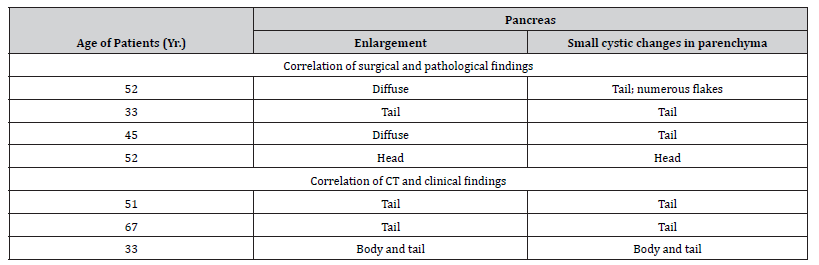
All seven patients were male with a mean age of 52 years. CT scans of the seven patients were analyzed for the degree and pattern of dilatation of the intrahepatic bile ducts; degree of dilatation of the common bile duct and the gallbladder and their intraluminal pathologic change: degree and pattern of enlargement of the pancreas, dilatation of the pancreatic duct and gross morphologic change. The CT findings of all seven patients are summarized in (Table 2). Overall, based on the CT findings of patients with Clonorchis sinensis pancreatitis Kim et al. demonstrated that presence of diffuse mild intrahepatic bile duct dilatation, the enlargement of the body or tail (or both) of the pancreas with a cluster of small cystic changes within the pancreatic parenchyma was strong evidence for the possibility of C. sinensis pancreatitis [54].
Table 2: Summary of CT characteristics in patients with Clonorchis sinensis pancreatitis.
Furthermore Lim, et al. reported that marked tortuous dilatation of the tributary ducts of the tail of the pancreas with atrophy of the pancreatic parenchyma was identified in patients with C. sinensis pancreatitis [50]. Balthazor and Lamb reported that the enlargement of the entire pancreas was depicted and was associated with peripancreatic fluid collection with the development of calcific pancreatitis on a follow-up examination 3 years later [55].
Furthermore Lim, et al. reported that marked tortuous dilatation of the tributary ducts of the tail of the pancreas with atrophy of the pancreatic parenchyma was identified in patients with C. sinensis pancreatitis [50]. Balthazor and Lamb reported that the enlargement of the entire pancreas was depicted and was associated with peripancreatic fluid collection with the development of calcific pancreatitis on a follow-up examination 3 years later [55].
Conclusion
Data on pathophysiology involved with various parasites causing AP is limited. The current review article was an attempt to summarize the isolated cases worldwide reported with regards to AP. Thus far, evidence in literature is limited to multiple case reports and retrospective studies. Advanced diagnostic techniques like a biopsy or fine-needle aspiration, along with tissue culture, PCR, or in-situ hybridization, are required to give us a better understanding of the role played by infectious agents in causing AP. Acute pancreatitis secondary to malaria is a very rare complication, both are life-threatening conditions and require critical care and close monitoring. When they occur simultaneously it poses a greater challenge for physicians. Hence, being vigilant to this rare but dreaded complication of falciparum malaria can help the clinician to detect, treat as well as prognosticate the patient efficiently.
To read more about this article...Open access Journal of Gastroenterology & Hepatology
Please follow the URL to access more information about this article





No comments:
Post a Comment