Authored by Ebuehi OAT*,
Abstract
Fruits detached from the living plants cannot repair its cells or defend its tissues against natural deterioration. Pectinases, the first enzymes to be secreted by fungal pathogens, weakens the plant cell wall and expose other polymers to degradation by hemicellulases and cellulases, are of significant importance in current biotechnological era, with their applications in various industries. The objective of this study, is to isolate and characterize pectinase producers from Bacillus subtilis and Aspergilus niger based on their carbon and nitrogen sources, using different assays. Fresh and rotten orange and pineapple peel were incubated in nutrient broth and the resulting microorganisms Bacillus subtilis and Aspergilus niger were subculture in pectin agar. Pectinase was extracted with pectin agar incubated at room temperature for 6 days. Clear zones of pectinase were isolated for secondary screening by estimating polygalacturonase (PG) and pectin lyase (PL) production under solid state fermentation by using the modified medium. Pectinase activity was analysed by alternating certain parameter, such as temperature and pH. Concentration of D-galacturonic acid and polygalacturonase were adjusted to investigate pectinase activity. The effect of inoculum size (106 CFU/ml, 105 CFU/ml, 104 CFU/ml and 103 CFU/ ml) for the production of pectinase. From this study, 104 CFU/ml and 103 CFU/ml were optimum inoculum sizes for the production of pectinase for both rotten and fresh oranges and pineapples respectively with level of significance (p < 0.05). Optimum pectinase activity was recorded at pH 5 and temperature 35 °C and 30 °C and pH5 and temperature 30 °C and 35 °C for both fresh and rotten oranges and pineapples respectively. Considering its great significance, with tremendous potential pectinase offers industries, one can conclude that strategic ways to produce maximum pectinase with low cost production should be studied
Introduction
When fruit is detached from the living plants it cannot repair its cells or defend its tissues against natural deterioration. There are many reasons of fruit destruction, but main cause of fruit spoilage is invasion by microorganisms such as mould, yeast and bacteria. Fruits contain high levels of sugars and nutrients and their low pH make fruits particularly desirable to fungal decay [1]. During postharvest handling, mechanical damage causes the release of enzymes present in the cell tissues. These enzymes break down the cellular material. Pectinases are the first enzymes to be secreted by fungal pathogens when they attack plant cell walls, weaken the plant cell wall and expose other polymers to degradation by hemicellulases and cellulases [2]. Pectinases are one of the most widely distributed enzymes in bacteria, fungi and plants insects, nematodes, plants, and protozoan [3]. Fungi are considered to be prospective enzyme producing sources.
Pectinases breaks down pectin and are of significant importance in the current biotechnological era with their all-embracing applications in fruit juice extraction and its clarification, scouring of cotton, degumming of plant fibers, waste water treatment, vegetable oil extraction, tea and coffee fermentations, bleaching of paper, in poultry feed additives, in alcoholic beverages and food industries [4]. Pectinases constitute approximately 10% of the total enzyme production in the world market and 25% of global sale in the food industry [5].
In the fruit juice industry, they are used for clarification of fruit juice by reduction in viscosity, increasing of juice yield by enzymatic liquefaction, and maceration of pulps, enhancing pigmentation by extracting more anthocyanin [6]. Microbial pectinases have been widely studied due to the requirement of highly productive strains and the cost-effective production of enzymes. In the present study, the pectinase was purified from a newly isolated Bacillus subtilis strain and this enzyme was characterized Identification and characterization of various pectinase producers have been published. There is paucity of information from the literature on combined action of two fruits and their mode of action. The aim of this study is to isolate and characterize based on primary and secondary screening, pectinase producers and how to optimize them from Agro-wastes, orange and pineapple
Materials and Methods
Reagents
The reagents and chemicals used in the study include: Pectin, K2HPO4, MgSO4.7H2O, NaCl, CaCl2.2H2O, FeCl3.6H2 O, yeast extract, Agar, urea, (NH4)2SO4, MgSO4, FeSO4, and glucose, were all of analytical grade and were purchased from Hi Media and Sigma Chemicals, USA.
Materials
Collection of samples
The fresh oranges were plucked from a tree in Alagomeji, Lagos, Nigeria. The fresh pineapples were gotten from a pineapple plantation in Epe, Lagos. The oranges and pineapples which were intended to be rotten were purchased from Mushin market, Lagos, Nigeria. The rottenness of the oranges and pineapples was obtained by leaving the purchased fruits for 14 days on a flat surface in the laboratory.
Isolation of pectinolytic fungi
Fungi were isolated from the rotten fruits (oranges and pineapples) by using the modified pectin agar medium. In brief, two-fold dilutions of the spoiled samples were plated in pectin agar medium and incubated at room temperature for 6 days. The strains grown in the plate were subcultured into pectin agar medium separately and flooded with 1% cetrimide for primary screening. The pectinolytic fungi produced a clearing zone when exposed to cetrimide [7].
Secondary screening
The fungal strains which showed clearing zones in pectin agar medium were selected for secondary screening by estimating the polygalacturonase (PG) and pectin lyase (PL) production under solid state fermentation by using the modified medium [8].
Isolation of pectinolytic bacteria
The isolates of both the rotten and fresh pineapple were cultured and incubated at 35°C for 48 h. Thereafter, the distinct bacteria colonies were streaked on nutrient agar and incubated at 35 °C for 24 h. When Gram-stained films were examined, the isolates that appeared as Gram-positive rods was identified as Bacillus subtilis based on result from biochemical test as enumerated [9-10]. The Bacillus isolates were maintained in nutrient agar slants at 4°C.
Enzyme assays
Polygalacturonase activity was measured according to the method described by [11-13] One unit of enzyme activity has been defined as the amount of enzyme that releases one μ mol of galacturonic acid /min under the assay conditions [12-13]. Pectin lyase activity was measured by the reaction between unsaturated end products of pectin degradation and thiobarbituric acid. One unit of activity is the amount of enzyme causing a change in absorbance of 0.01 under the conditions of the assay [14].
Effect of inoculum age, inoculum size, and incubation time
Inoculum age was optimized by inoculating the medium (secondary culture) with inocula (primary culture) of varying age, namely 8, 12, 16, 20, and 24 h, and assaying the filtered broth for enzyme activity. To study the effect of inoculum size, (103-106CFU/ ml) inoculum was used to inoculate the production medium, and polygalacturonase activity was assayed in the broth. The effect of incubation time was studied by incubating the microorganism in production medium for different time intervals (24, 48, 72 and 96 h) and measuring the enzyme activity.
Effect of temperature and pH
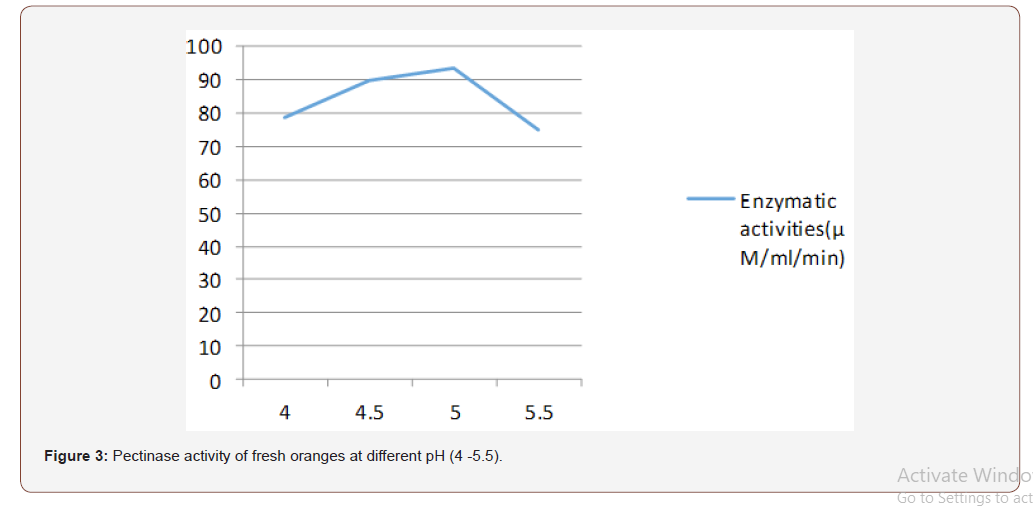
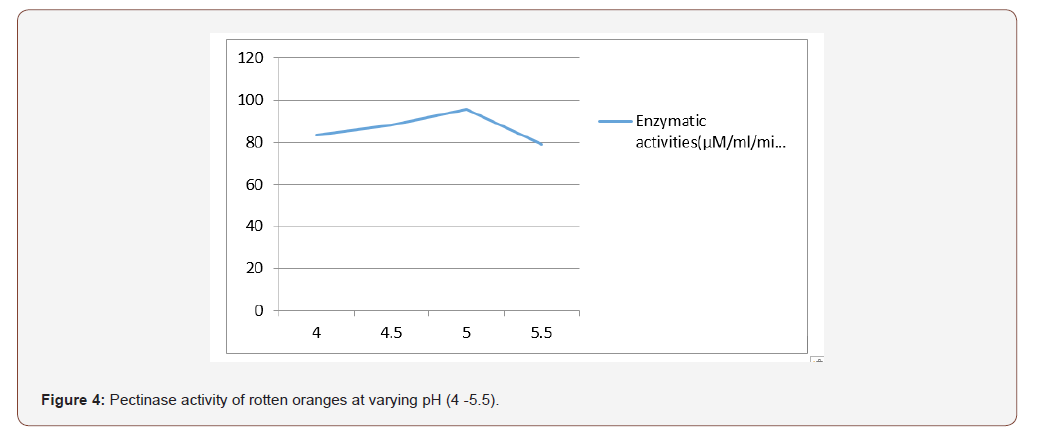
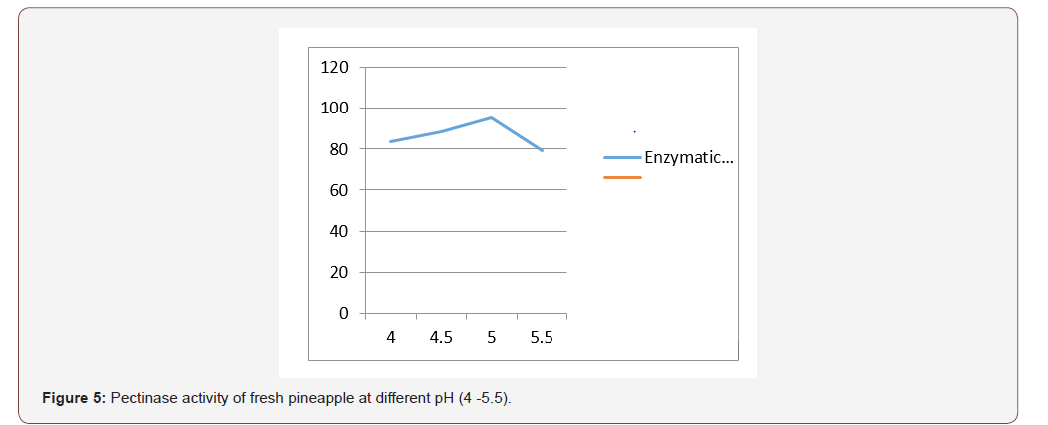
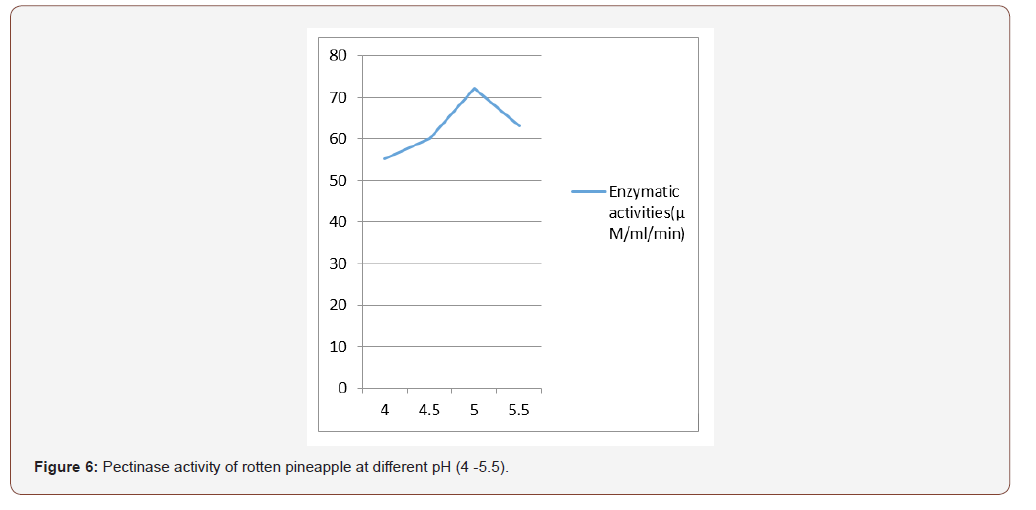
Most favorable production temperature was studied by incubating the production medium at different temperatures (20, 30, 35 and 40 °C). Polygalacturonase activity was assayed by using the standard method [11]. For optimizing the production of pH, the production medium varying pH, namely, 4.0, 4.5, 5.0, 5.5, was used for enzyme production, and activity was measured.
Effect of nitrogen source
The effect of various nitrogen sources (Ca (NO3)2, (NH4)2SO4, (NH4)2NO3, NH4Cl, NaNO3, KNO3, and Urea on the production of enzyme was studied by supplementing 0.1% w/v of these to the production media.
Effect of D-galacturonic acid on polygalacturonase production
To study the effect of D-galacturonic acid on polygalacturonase production, it was added to the culture broth at a final concentration of 0.3, 0.6, 0.9, 1.2, and 1.5%w/v under aseptic conditions. The resulting extracellular polygalacturonase activities produced were measured by estimating the reducing groups produced (Figures 1-6).

Discussion
Pectinolytic enzyme can be derived from different sources [15-17]. However, pectinase producing microorganisms have due advantage over other sources because they can be subjected to genetic and environmental manipulations to increase yield [18]. It has been reported that most Bacillus sp, enhances the production of pectinase [19]. This result indicates that orange and pineapple contain appreciable amount of pectin. The pectin was used as inducer for the production of pectinolytic enzymes. In this study, pectinase production by Bacillus subtilis and Aspergillus niger were compared. Our results show that both Bacillus subtilis and Aspergillus niger were able to produce pectinase. The isolates were later designated based on morphology as Bacillus subtilis, and Aspergillus niger are among the most studied pectinolytic fungi (Sukumaran et al., 2005; Favela-Torres et al., 2006). [20] also has reported that Bacillus sp, has produced higher yield of pectinase compared to most other microbes such as Aspergillus.
Temperature is very important factor for microbial growth as well as microbial product formation. The incubation temperature greatly affects the microbial growth rate, enzyme secretion, enzyme inhibition, and protein denaturation. Thermal stability and activity of pectinases are of great significance in biotechnological process. In this study, it was reported that 30 °C was the optimum incubation temperature for fresh and rotten orange and fresh pineapple but 35 °C was observed as the optimum incubation temperature for rotten pineapple. In previous report on A. niger, using orange and pineapple D-galacturonic acid as carbon source exhibited maximum activity at 35 °C which was in accordance to findings by [21].
The initial pH of the fermentation medium plays a vital role in determining the level of metabolite synthesis. The stability of the microbial metabolite is also dependent on the hydrogen ion concentration of the medium [22]. In this study it was found that the optimum pH condition for the production of pectinase is pH 5 for fresh and rotten orange and pineapple respectively. According to Spagna et al., 1996, maximum pectinase activity was under pH 5. The optimum pH from this study is comparable to pectinase of Penicillium varidictum and Penicillium oxalicum [23-25]. [26] reported that optimum pH 5.5 for pectinase activity from thermotolerant Aspergilus sp.
Maximum pectinase activity was observed with 104 CFU/ml and 103 CFU/ml inoculums size for both fresh and rotten oranges and pineapples respectively. The effect of inoculum sizes for the production of pectinase fresh and rotten pineapple respectively. Optimum inoculums density is an important consideration for fermentation process since accumulation of spore can inhibit growth and development of the culture organism [27].
An adequate supply of carbon as energy source is critical for optimum growth of organism and its metabolism. In the present study, supplementing carbon sources increased pectinase production on solid state. According to [28] low enzyme production with other carbon sources might be because of catabolite repression. Glucose is known to repress the transcription of genes encoding enzymes required for the utilization of alternative carbon sources; some of these genes are also repressed by other sugars such as galactose, [29].The effect of D-galacturonic acid with various concentrations (0.3%, 0.6%, 9%, 1.2%, 1.5%) was evaluated in this study and it was observed that there were increase in the pectinase activities with increase in the various concentrations of the D-glucoronic acids as [29] found that pectin and polygalacturonic acid promoted the production of pectic enzyme.
The effect of nitrogen sources was also observed in this study for both rotten and fresh oranges and pineapples. Of the various nitrogen sources used, maximum pectinase activity was observed when medium was supplemented with NH4Cl for the whole samples. Meanwhile, among the tested nitrogen sources, ammonium sulphate (NH4SO4) and ammonium nitrate (NH4NO3) increased the pectinase productivity on SSF though their effect was not significant. The result is in agreement with [30], who found that ammonium sulphate and ammonium nitrate were good nitrogen sources for pectic enzyme production from A. niger. Moreover, Sarvamangala et al., (2006) revealed that ammonium sulphate did influence production of pectinase positively in solid-state conditions.
To read more about this article...Open access Journal of of Agriculture and Soil Science
Please follow the URL to access more information about this article
To know more about our Journals...Iris Publishers
To read more about this article...Open access Journal of of Agriculture and Soil Science
Please follow the URL to access more information about this article
To know about Open Access Publishers





No comments:
Post a Comment