Authored by Sander BQ*,
Chemical aspects and mechanisms of action
Sucralfate is a complex salt of sucrose sulfate and aluminum hydroxide. In it, the sulfated disaccharide is formed by the combination of a sucrose octosulfate plus aluminum hydroxide1 (Figure 1). This substance is a cytoprotector and is used for the most diverse conditions and gastrointestinal diseases [1, 2], with reports of use also for various skin conditions [3-5].
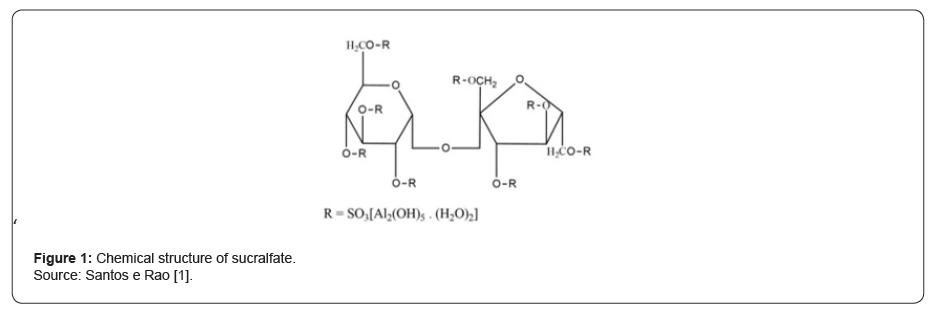
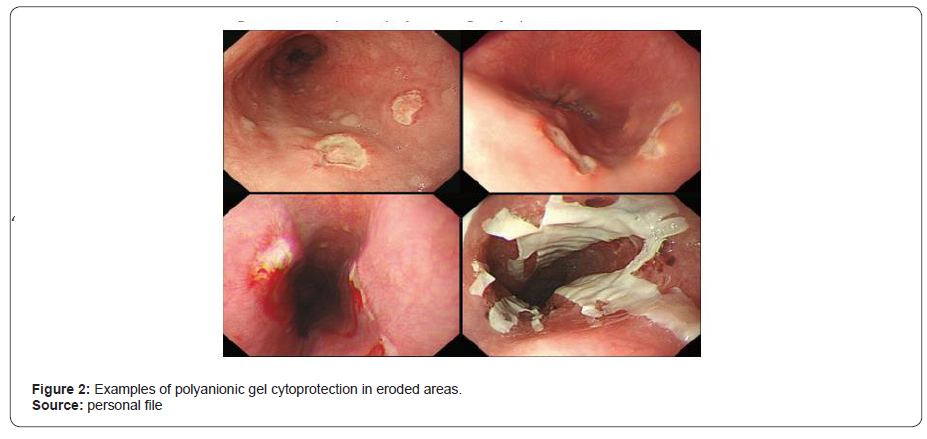
The cytoprotective action, as indicated by Santos and Rao [1], results from several characteristics of sucralfate. Due to its low solubility in water, when dissolved in an acid or alkaline medium, it is dissociated into aluminum salt and sucrose sulfate, becoming like a polyanionic gel when it binds, with high affinity, to normal or eroded mucosa. Due to these characteristics, with the action of gastric pH, negative aluminum is released, and it is electrostatically prone to bind to positively charged chemical groups. In the case of its cytoprotective action, the transformed compound binds to proteins, peptides, among others, forming a glycoprotein complex that covers the damaged surface and will provide cytoprotection to that area [5, 6] (Figure 2).
In the gastroesophageal tract, the formation of this physical barrier protects against acid, pepsin, and bile salts, in addition to increasing mucus production. It also inhibits the interaction between pepsin and its substrates, stimulates the production of prostaglandins by the mucosa and adsorbs bile salts, without interfering with acid production or gastrin secretion [7]. In other words, the most reported therapeutic action of sucralfate is the result of three mechanisms: (a) local action on areas of ulcerated mucosa forming a protective barrier that reduces pepsin activity and H + injury; (b) action on pepsin chelation and bile salts; and (c) mucosal-wide trophic effect that facilitates both mucosal healing and re-epithelialization.
Dosage and Administration
The drug is usually administered orally, in chewable tablets or vials, in possible different concentrations. In general, for adults, the recommended dose is 1 chewable tablet (1g) four times daily or 2 chewable tablets twice daily, on an empty stomach, one hour before or two hours after meals. The vials (2g) have the same recommendations, except for their administration, necessarily three times a day. And, given its low absorption (0.5 to 2.2% of the dose), its therapeutic effect lasts for around 6 hours.
Research and reports on the clinical use of sucralfate
In Brazil, the pharmaceutical and therapeutic version of sucralfate is Sucrafilm®, with general indication for the treatment of duodenal ulcer, gastric ulcer, acute or chronic gastritis, heartburn, heartburn, and dyspepsia. There are, however, several reports about its possible uses, indicating great potential for the substance, in addition to those already explored. Teng et al8 investigated the efficacy of sucralfate combination therapies on Helicobacter pyloriinduced gastric mucosal injury and its effect on the gastrointestinal flora, with interesting findings. Triple antibiotic therapies associated with the use of sucralfate effectively eradicated H. pylori infection, showing protective effects against histological damage induced by the bacterium.
Santos and Rao [1] showed that its clinical use is indicated for the prevention or treatment of gastroesophageal reflux disease, peptic ulcer disease and dyspepsia, in addition to having been useful in patients with ulcerative colitis. Kudaravalli and John [2] reported its use for duodenal ulcers, but also for various epithelial wounds, chemotherapy-induced mucositis, radiation proctitis, ulcers in Behcet’s disease and burns. The use of Sucrafilm® was reported by Sander, B.Q. in Endoscopic Sleeve Gastroplasty (ESG), in post-procedure complications, demonstrating significant results in the reduction of bleeding after the procedure and in the highest percentage of fibrosis in the gastric tubulization.
Dagli and Kalkan [9] reported, among the various therapeutic options available, the possibility of safe use of sucralfate in pregnant women with gastroesophageal reflux disease (GERD), with considerable improvement in heartburn. The authors also suggest its use in cases of GERD during the lactation period, since the systemic absorption of the drug is minimal, which reinforces the safety of the drug. Previous studies also demonstrate this assertion. One of them, on the safety of sucralfate use in pregnancy, with 42 women who received doses of sucralfate 3×1 g/day, for one month, showed that there were no maternal or fetal side effects [10]. Another observational study, with 229,101 pregnancies followed between 1985 and 1992, showed negligible rates of birth defects in newborns who were exposed to sucralfate1 [1].
Chatterjee et al3 indicated its topical use in skin ulcerations, showing the healing effect of topical sucralfate and increased antimicrobial effect of mupirocin for the general improvement of chronic dermal ulcers. Proposals such as this had already been explored, such as the formulation of a topical preparation of sucralfate to be used in the healing of skin wounds, as an antiseptic, in the application of drugs with topical activity (dermal and mucosa) and for the formulation of beauty masks and other cosmetic preparations [5].
Along these lines, Ribet, et al. [4] proposed a new dermocosmetic product containing thermal water, sucralfate, copper sulfate and zinc sulfate in the treatment of hand eczema. Product tests showed improvement after 7 days of application, thus suggesting its use for contact dermatitis and climate dermatitis.
Topical use seems to be one of the most proposed lines of research on the possible uses of sucralfate. Sato et al6, based on the work of Masuelli, et al. [5], noted that sucralfate promotes tissue healing, increasing the production of epithelial growth factor, exhibiting anti-inflammatory properties, and providing antioxidant activity. These characteristics are highlighted by Masuelli, et al. [5] when exploring the literature on the topic, endorsing the topical use of the substance. Some time ago, Masuelli, et al. [5] had already endorsed this use through literature studies. Thus, the use of sucralfate as a topical agent has been suggested, alone or in combination with other drugs, to treat the most diverse diseases. Among other possible recommendations, with greater or lesser scientific evidence, authors cite: anorectal diseases and in particular hemorrhoids or other dermal wounds; aphthous ulcers and other mucosal, submucosal, epidermal, dermal or subcutaneous tissue; dermal ulcers such as bedsores, stomatitis; alopecia; symptoms of herpes, acne, psoriasis, eczema, bed sores, shingles, itchy skin, athlete’s foot, ringworm and other dermal conditions; irritation and discomfort associated with burns; skin damage including inflammation, infection or burns and rosacea when used in combination with other drugs [5].
More recent studies reinforce the use of sucralfate in diabetic ulcers, one of the most serious and devastating complications of diabetes mellitus. This situation creates difficulty in treatment and often results in loss of limbs. One study, although conducted in the laboratory and in non-humans, showed that the combination of topical sucralfate and platelet-rich plasma therapy results in improved ulcer healing, compared with sucralfate or platelet-rich plasma monotherapy, or conventional therapy with wound healing. The wound protection and repair mechanism of sucralfate appears to be related to its ability to maintain blood vessel integrity. As one of the main pathological mechanisms of diabetes mellitus is vasculopathy, sucralfate would help in maintaining adequate blood flow, thus improving the bioavailability of wound healing growth factors [12]. The results of this research also seem to follow the clinical evidence noted by Nagalakshmi, Amalan and Anandan [13] regarding the topical use of sucralfate in the healing of epithelial wounds.
Risks, adverse effects, and recommendations
The risks associated with sucralfate overdose are minimal, as sucralfate has very little absorption by the gastrointestinal system [2]. In general, the literature on the subject [1, 2, 7] is consensual in showing that the drug is safe and well tolerated. In clinical experiences, adverse reactions to sucralfate are minimal and rarely lead to the need to discontinue the drug, its main adverse effect being constipation. The rate of this complication is variable, ranging from 2 to 5% of patients. Other adverse effects include dry mouth, nausea, vomiting, headache, urticaria and skin rash [1]. Sucralfate has several drug interactions and can decrease serum concentrations of digoxin, levothyroxine, furosemide, quinolones, oral phosphate supplements, warfarin, antiretroviral drugs such as raltegravir, bisphosphonates, among others, and administration of the drug at 2-hour intervals between these is recommended medicines [2].
Constipation, which appears to be linked to Al3+ (in 2% of cases), and dry mouth sensation (< 1%), are significantly related to the drug. There are occasionally patients with reports of abdominal discomfort, but without significant relationships. The effects on phosphate metabolism in plasma Al3+ are similar to those already described for Al(OH) [3]. Recently Kuradavali and John [2], among their objectives, sought to identify both the mechanism of action of sucralfate and to describe its potential adverse effects and toxicity. They reinforced the factor of almost insignificance of side effects of the product as a safety parameter, but emphasized the constipation rate of 1 to 10%, according to the suggested studies. The authors noted reports of hyperglycemia in diabetic patients using sucralfate, among other side effects of little relevance. In addition, inadvertent use of intravenous sucralfate has caused fatal complications such as pulmonary embolism and cerebral edema. The contraindication is, above all, due to hypersensitivity to sucralfate, with relative caution in cases of end-stage renal disease, uncontrolled diabetes mellitus with hyperglycemia and impaired swallowing reflex [2].
To read more about this article...Open access Journal of Gastroenterology & Hepatology
Please follow the URL to access more information about this article





No comments:
Post a Comment