Authored by Joseph W Eichenbaum*,
Abstract
Glaucoma, the second leading cause of blindness worldwide is a neurodegenerative disease, with or without elevated ocular pressure, exhibiting several different phenotypic presentations, which result in progressive visual field loss after compromise of retinal ganglion cells and their axons to the optic nerve and brain. Predominant clinical glaucoma types are primary open angle, POAG, angle closure, and congenital; secondary glaucomas are: psuedoexfoliaiton, pigmentary, neovascular, traumatic, iridoendothelial, and uveitic glaucoma. In each of these types of glaucoma, genetic, epigenetic, proteomic, environmental, and mechanical forces may converge as well to cause or advance the disease. Alternatively, but not necessarily in an independent fashion, stress related pathways of oxidative stress, inflammation, infection, trauma, immune reaction, neuro-degeneration and mutant genomic products can produce deleterious misfolded proteins, antibodies, and aggregate deposits in the trabecular meshwork, TM, raise the intraocular pressure and result in optic nerve damage and loss of vision. Examining the interplay of these stress pathways with genetic, epigenetic, proteomic, environmental and mechanical forces to gain some understanding of molecular causes of glaucoma is the goal of this paper
Keywords: Glaucoma GWAS, Myocilin mutants/misfolded proteins, Oxidative stress, HSP, Inflammation, Immune/autoimmune response, Mitochondrial lysosome axis dysfunction, Autophagy pathways, Trabecular meshwork ECM dynamics, RGC/brain neuroprotection, Epigenetic role
Abbreviations: AH: Aqueous Humor; ATP: Adenosine Triphosphate; Bcl-2: B cell lymphoma 2; CHOP: A multifunctional transcription factor in endoplasmic reticulum oxidative stress and protein misfolding; CLAN: Cross linked actin network; DARC: Detecting apoptosing retinal ganglion cell technique; ECM: Extracellular matrix; ELAM: Endothelial leukocyte cell adhesion molecule; EM: Electron microscopy; ER: Endoplasmic reticulum; GWAS: Genome wide association study; HSP: Heat shock protein; IOP: Intraocular pressure; JCT: Juxtacanalicular region of the trabecular meshwork; JNK: Jun N-Terminal Kinase; MAGP-1: Microfibril associated glycoprotein; MAP: Kinase mitogen activated protein kinase, increase ECM production in TM cells; ERK: Kinase extracellular; MAP: Microtubular kinase; MIP: Macrophage inflammatory protein; MITF: Microphthalmia associated transcription factor; MMP: Matrix metalloproteinase; MTORC-1: Mechanistic target of rapamycin complex overseas protein synthesis for Lysosome function; Myoc-OLF: Myocilin olfactomedin domain five-bladed β-propeller protein family; NAD: Nicotinamide adenine dinucleotide; NFKB: Nuclear factor kappa-light-chain-enhancer of activated B cells, major transcription factor for inflammation/immune modification in the cell; NMDA: N-methyl D Aspartate; NTG: Normal tension glaucoma; Nrf2: Nuclear factor erythroid 2, major oxidative stress transcription factor; PDS: Pigmentary dispersion syndrome; PERG: Pattern electroretinogram; PG: Pigmentary glaucoma; PI-3: Kinase phosphoinositide-3 kinase; PMEL: Premelanosome protein that may form physiologic amyloid beta protein in melanosomes; POAG: Primary open angle glaucoma; PTEN: Phosphatase and tension homologue; PTP: Mitochondrial permeability transition pore; PXG: Pseudoexfoliaiton glaucoma; PXS: Pseudoexfoliaiton syndrome; REST: Repression element silencing transcription factor; Retinal Ganglion Cell; Rho Kinase protein kinase modifying cell shape, size and adhesion; SC: Schlemm’s Canal; SPARC: Secreted Protein Acidic Rich in cysteine, binds to ECM proteins and regulate matrix metalloproteinase expression; SMAD: ‘Small Mothers against decapentaplegic homolog’ literally but actually transcription factor which mediates TGF beta transduction or simply helps transfer cytoplasmic signals to nucleus to activate molecules like MAP or Rho kinase; TEK: Receptor tyrosine kinase; TGF: Beta transforming growth factor beta (signalling differentiation, proliferation, chemotaxis and fibrosis); TIMP 3: Tissue inhibitor of metalloproteinase; TLR4: Toll like receptor 4; TM: Trabecular meshwork; TNF: Tumour necrosis factor; TNFR: Tumour necrosis factor receptor; UPR: Unfolded protein response; VCAM1: Vascular adhesion protein; VEP: Visual evoked potential
Introduction
Glaucoma is a complex disease with many different phenotypes that can manifest over a lifetime. Childhood glaucoma, for example, also referred to as congenital or infantile glaucoma occurs in babies and young children. Although it is a rare condition, it may be inherited and is usually diagnosed within the first year of life by the elevated ocular pressure, abnormal drainage angle structure and excavated optic nerve. It develops from improper development of the eye’s TM/SC (trabecular meshwork/Schlemm’s Canal) drainage system before birth [1].
By comparison, recent work has suggested that typical alterations for open angle glaucoma, POAG, one of the leading causes of irreversible worldwide blindness, were observed in a subset of ocular normotensive Alzheimer’s disease patients on Heidelberg Retinal Tomograph-3, (which photographically and statistically monitors: linear cup/disc ratio, cup shape measurement, rim area, rim volume, height variation contour, and mean retinal nerve fiber layer (RNFL) thickness) [2].
Thus, over a lifetime, different pathways from within the two large glaucoma defect categories of impaired aqueous outflow channels and nerve damage to percipient and conductive neural elements of the eye and brain have been implicated in the neurodegenerative glaucoma process [3].
From a historical perspective, trabecular meshwork, TM/ Schlemm’s canal, SC, defects were primal to the development of elevation of intraocular pressure and optic neuropathy. However, in the last two decades the narrative has become more detailed with the elucidation of glaucoma category genes, proteins, and transcription factors in both the TM, and retinal ganglion cell, RGC, anatomic regions. These glaucoma category details encompass the language of RGC and TM cell survival after sustained oxidative stress from mutant derived and misfolded protein challenging mitochondrial sufficiency, provoking immune and inflammatory reaction, and leading to either repair/maintenance or apoptosis and cell death.
In this article I would like to first highlight some of the salient genes and proteins from each of these categories. SIX6, (retinal ganglion cell related), FNDCB3, FMNL2, (IOP, POAG related), CDKN2B-A (vertical cup/disc related), PLEKHA7, HGF, FERMT2, and GLIS, (narrow angle glaucoma related), ANGPT1, ANGPT2 and VEGF-C, (Schlemm’s canal regulation through receptor tyrosine kinase), LOXYL1 (through the window of pseudoexfoliaiton glaucoma), and PMEL, ( a protein related to pigmentary glaucoma). These elements constitute the first section of the paper.
POAG, IOP, Cupping of the optic nerve, visual field defects, TM/Schlemm’s canal outflow issues, pseudoexfoliaiton glaucoma and pigmentary glaucoma are the basic vocabulary of the ophthalmologist in first considering a diagnosis of glaucoma. Thus, genomic and proteomic correlates to these glaucoma characteristics and glaucoma types are discussed second.
Characteristic proteins from the aqueous humor, AH, of glaucoma patients, associated with cell signalling, glycosylation, immune response, molecular transport, and lipid metabolism initiates the global protein or second sub-section of the paper.
Then, proteins of the TM extracellular matrix, myocilin, TGF beta, clusterin, amyloid, heat shock proteins, Toll receptors, interleukins, and ubiquitins are reviewed. In their individual and concerted efforts in RGC and TM these proteins constitute a pervasive framework for advancing or retarding glaucomatous damage.
In the third section, the mechanism of glaucoma elements as seen through the windows of the proteomics of adult prevalent: pseudoexfoliaiton, primary open angle, and pigmentary glaucoma are reviewed. Incriminating data on mutated/misfolded myocilin, (the first gene linked to glaucoma) and its contributors to compromised AH outflow and their repair pathways and their alternate roads to apoptosis are explored.
That said, it is worth keeping in mind that enhanced differential gene/protein expression levels of these (and other glaucoma genes) do not necessarily dictate a linear relationship to their functional outcomes. Thus, the concluding sections of this paper examine other dynamic vectors which characterize the somewhat hidden, non IOP elements of the disease: heat shock proteins, (with their immune clearance effect or their potential axonal cushioning effect), nitrous oxide donating moieties for increased AH outflow, glaucoma signal pathways, epigenetic and other modifiers for neuroprotection vs neurodegeneration in glaucoma. Such signal vectors as TGF beta, for example, can produce excess ECM, extracellular matrix protein, in the TM which can block AH outflow. But other transcription factors, such as MAP kinase can initiate a process for removal of aberrant proteins. Certain inflammatory factors can overload the clearance of misfolded proteins and other epigenetic chromatin modifiers can activate genes for alternate pathway clearance. The analysis of interactions of fortifying and debilitating vectors and their outcomes constitute the next platform for glaucoma therapy.
Thus, these vectors as well as aging, oxidative stress, protein misfolding, mitochondrial lysosome axis dysfunction/repair are all on a dynamic, wide stage of activity (for TM and RGC categories of glaucoma) with many actors in a comprehensive, ongoing dialogue of intracellular homeostasis, survival vs. glaucomatous damage and apoptosis.
Outline
POAG
GWAS
Genes Clinical Features
PXG Proteins & Features
AH Proteins in POAG
Myocilin Protein Changes in Glaucoma
Pigmentary Glaucoma
POAG TM/RGC: Ox. Stress,
>energy demand
on mitochondria,
Heat Shock Proteins:
Inflammation, immune,
autoimmune reaction;
> damaged proteins> Ubiquitin activity
Apoptosis
positive correlation:
mean visual field deviation
oxidative stress
Nitrous Oxide> AH outflow
Glaucoma Signal Paths
Neuroprotection vs
Materials and Methods
To obtain a broad spectrum of papers in genomics, proteomics, epigenetics and signal transduction pathways of glaucoma, each of these topics was named in association with glaucoma and searched in pub med and Google Scholar over the last two decades up to the year 2020. Papers that provided a systematic review with clear supporting evidence for associations of glaucoma to evidence-based mechanisms such as: genes from genome wide association studies, protein misfolding as a consequence of e.g. myocilin mutations, the role of oxidative stress in mitochondrial/ cytochrome demise and apoptosis or E-3 ligase involvement in clearing dysfunctional protein were given greater weight as were their supporting reference papers. In this regard, papers that linked mechanistic findings with risk elements in glaucoma, such as visual field loss or vertical cup disc ratio were also weighted heavily in the initial selection.
Results
POAG Genome wide association studies, GWAS, ethnic genetic associations and genes associated with glaucoma clinical features
GWAS, have documented some 74 genomic loci that have been associated with POAG susceptibility. Some of these characterize ethnic specific risk factors as well as risk factors across diverse ancestry [8].
In the age groups, 40-80 years, the global prevalence of POAG is 3.54%. In those of African descent, it is even higher at 4.2%. Worldwide in 2020 it was estimated to be a total of 64.3 million with glaucoma [5]. While elevated intraocular pressure, IOP, may or may not be associated with glaucoma, early on patients are often asymptomatic or only experience mild peripheral or central visual field disturbance, usually after silent optic nerve micro hemorrhage, ischemia, oxidative stress, inflammation, and immune reaction has caused some damage [6,7].
Genome- wide association studies in POAG over the last decade have identified risk loci in European, Asian, Japanese and African populations [8]. Individuals of African ancestry have three to five times the POAG risk and worse visual field and disease progression than other populations [8-13]. Thus far, as is the case with Japanese populations, it is unclear if Asian study gene markers for the Japanese are able to be associated with other Japanese study loci, and, if loci from the African populations are compatible with studies of loci of African Americans [8,10-14]. However, Hispanic/ Latino populations, although much less explored in GWAS, have illustrated loci previously identified in GWAS of European or Asian populations in their Hispanic/Latino sample [8]. These and other genetic ethnic loci variances in different studies illustrate the genetic heterogeneity across populations. Other studies in glaucoma clinical features, such as intraocular pressure and vertical cup to disc ratio, have been examined with respect to genetic locus and potential POAG risk. Patients with these features and genetic loci may be higher risk and may require earlier intervention.
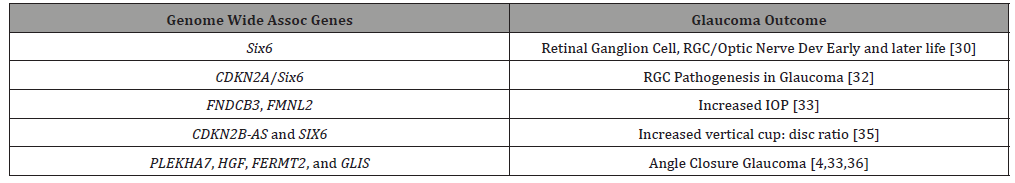
Unfortunately, thus far GWAS have not provided a specific overriding genetic mechanism for understanding glaucoma. However, certain POAG gene candidates have led to further investigation. For example, SIX6 (which is important in retina and optic nerve development) expressed abnormal gene variants and illustrated reduced protein elaboration in zebrafish in vivo assays, [15- 18]. With abnormal human homozygous SIX6 alleles vs normal homozygous alleles, the nerve fiber layer was thinner in the abnormal SIX6 homozygous population. The RGC are the primary target in glaucoma [31]. Moreover, a SIX6 gene variant showed increased expression of another POAG normal tension glaucoma locus, CDKN2A, in cell lines, and in human glaucoma with increased IOP causing senescence in retinal ganglion cells [19].
Clinical features of glaucoma have been correlated to GWAS POAG gene loci. FNDCB3 and FMNL2 have been associated with increased IOP [20,21], and common loci variants CDKN2B-AS and SIX6 were linked to increased vertical cup disc ratio [22].
PLEKHA7, HGF, FERMT2, and GLIS, four loci conferring risk to angle closure glaucoma, were from the group with increased intraocular pressure, suggesting that angle closure may contribute to elevated IOP even in patients ostensibly within the normotensive range [8,20,23].
Corneal thickness glaucoma GWAS study association
A GWAS for central corneal thickness association with POAG found only one locus FNDC3B and surprisingly one central corneal thickness allele was found protective for POAG [24]. Another GWAS in over 25,000 European and Asian patients did not find any correlation between central corneal thickness and POAG or angle closure glaucoma [4,25]. This raises the question of whether the central cornea thickness-glaucoma criterium is in the same category as intraocular pressure, vertical cup to disc ratio, and optic nerve change phenotypes, but rather another risk factor as yet to be understood on the genetic/proteomic landscape [8]. (Table 1)
Table 1: Genome Wide Association Studies, Genes related to Glaucoma.
Glaucoma: GWAS hints at systemic disease, angiogenesis pathways/tyrosine receptor kinase schlemm’s canal IOP regulation; Glaucoma: Subacute bacterial inflammation (Toll Receptors) and Immune (Heat Shock Proteins, Toll Receptors in retinas with Glaucoma)
Despite the fact that GWAS have not provided clear cut gene to disease understanding, they have provided candidate genes for further glaucoma study in animal models and human cell lines and glaucoma association with systemic disease, such as type 2 diabetes and cardiovascular disease [8,31].
In addition, not unlike the GWAS highlighting Complement Factor H polymorphisms in macular degeneration, and systemic disease associations [26-30], GWAS in glaucoma may have begun to elucidate loci linkages to (1) glaucoma phenotypes, (IOP and vertical cup to disc ratio) and (2) to systemic diseases, such as diabetes, myocardial infarction and stroke [8,28]. In the latter, for example, from both IOP GWAS studies, genes involved in angiogenesis and “vascular endothelial cell morphology” emerged with ANGPT1, ANGPT2 and VEGF-C gene variants. These genes are TEK (receptor tyrosine kinase) signal regulators for Schlemm’s Canal modification of aqueous pressure. Mutations in TEK may cause primary congenital glaucoma [32]. Lesser alterations of the angiopoietin-TEK pathways may curtail efficient Schlemm’s IOP regulation. TEK receptors are highly expressed in Schlemm’s canal, [33]. In the mouse model, efficient maintenance of angiopoietin- TEK signalling may be responsible for protective VEGF-C anterior chamber levels and normal intraocular pressure at the Schlemm’s canal collector channels [34]. At this point, it is unclear if this VEGF-C IOP balancing/unbalancing may be in conjunction with TM dynamics in glaucoma, or, by itself a source of increased IOP.
Returning to the possible role of complement and immune response and inflammation in glaucoma (as in macular degeneration cited above) a recent study showed that glaucoma patients had higher bacterial oral counts compared to control subjects. Low dose bacterial lipopolysaccharide administration in glaucoma animal models developed enhanced axonal degeneration and neuronal cell loss. Microglial activation in the optic nerve and retina along with upregulation of TLR4 (Toll receptor 4) signaling and complement system, which was blocked by naloxone, suggested a bacterial/ infection, immune and possibly inflammatory role in glaucoma development [35].
In a related way, retinas from humans with glaucoma had parallel proteomic mass spectrometric and immunostaining for Toll Receptors 2,3,4. In line with upregulation of toll receptor signaling in the glaucoma samples, prominent expression increases in heat shock proteins were noted. Analysis of microglia and astrocytes in glaucoma vs control retinas also showed toll receptor expression increases. In vitro experiments after oxidative stress also showed heat shock protein increases in line with upregulation of glial Toll receptors and MHC Class II expression. Increased cytokine and T cell production were also linked to Toll Receptor upregulation [36]. These papers tie glaucomatous retinal tissue stress to upregulation of immune and inflammatory response [35,36].
Pseudoexfoliaiton glaucoma PXG, extracellular matrix, LOXYL1 gene, homocysteine, clusterin, misfolded protein, amyloid, hypoxia, chronic organic and brain disease association, life expectancy, hypoperfusion and elevated oxidative stress
Pseudoexfoliaiton syndrome (PXS), an aging disorder involving the extracellular matrix of the trabecular meshwork, ECM, (multiadhesive protein glycans, collagens, fibronectin, laminin, elastin, and others, secreted by TM cells for cell support, molecule migration, proliferation and differentiation) [37] is the most common risk factor for secondary glaucoma and a major cause of worldwide blindness. An estimated 60-70 million patients are affected [38-41].
A global sample of PXS identified new variants of LOXL1, a rare protective allele with greater cellular adhesion ability compared to the wild type and five new loci [42]. The protective LOXL1 in follow up histological investigation demonstrated decreased (1) elastic fiber formation, (2) tissue stiffness, and (3) cell adhesion in ocular tissues of patients with PXS [43].
LOXYL1 variation genes at the POAG locus have been associated with pseudoexfoliaiton glaucoma PXG. Of particular note, however, is that while the rare variant at LOXYL1 is protective, the five others nearby were only susceptibility loci. All five “LOXYL1 haplotypes detected by the resequencing effort showed reversal of genetic effect, their functional consequences remaining in doubt.” [42]. Although LOXL1 participates in crosslinking of collagen and elastin in the extracellular matrix, LOXL1 has not been specifically shown to cause pseudoexfoliaiton. Another gene, CACNA1A, however, which codes for a subunit in the voltage-gated calcium channels, (which are widely expressed in the brain and ocular tissues), through calcium deposition on electron microscopy in pseudoexfoliaiton, may be linked to pseudoexfoliaiton [43,44].
While the genomics of LOXL1 in pseudoexfoliaiton are as yet unclear, the role of lysyl oxidase, LOX, in the cell seems to be gaining greater understanding. Lysyl oxidase oxidizes the side chain peptide of lysine converting it to a semialdehyde which permits the covalent crosslinking of collagen chains and elastin. This stabilizes fibrous deposits and proteins in the ECM. Four LOXL proteins with varying similarity to LOX have been described. From this family of related proteins, a proLOX protein can emerge, which is activated to the enzyme lysyl oxidase, LOX.
LOX, in addition to overseeing crosslinking of collagen and elastin, plays a role in chemotactic responses, proliferation and shifts between normal and malignant phenotypes [45]. Thus, with the multifaceted and complex intracellular role of this gene/ protein family, the genomics await further clarification. This study, for example, demonstrated that some LOXL1 polymorphism alleles have different effects in different populations, with a given allele increasing pseudoexfoliation risk in certain populations but providing protection in others [46].
Increased levels of homocysteine in pseudoexfoliaiton patients in several studies may disrupt disulfide bonds of cysteine residues in extracellular matrix proteins. This homocysteine/ pseudoexfoliaiton may be relate to chronic folate deficiency which could disrupt the methione-homocysteine cycle and affect methylation of LOXYL1 and its expression [47].
Results remain mixed on the role of clusterin single nucleotide polymorphisms, SNPs. SNPs in some populations are positive and other populations negative for association to PXG. Clusterin, or apolipoprotein J, is secreted by almost all cell types and is named for its ability to cluster red blood cells. It is an extracellular matrix protein involved in reducing protein misfolding. Clusterin also binds amyloid and reduces amyloid formation in Alzheimer’s disease [48-50]. In the eye, studies examining common clusterin protein variants for association with PXS have been inconsistent [50]. It is of interest, that the literature has a report of a 70-year-old with PXG and lattice corneal dystrophy with amyloid accumulation [51].
In addition, clusterin because of its in inhibition of oxidative stress, induced aggregation and precipitation of misfolded proteins, and regulation of complement, may mitigate heat shock protein elaboration and resist apoptosis [52].
However, Zenkel et al showed reduced immunoreactivity for clusterin in ocular tissues of PXG eyes [53]. Patients with focal segmental glomerulosclerosis also have reduced blood and urine levels of clusterin [54,55].
Thus, there may be an initial beneficial clusterin level to reduce misfolded proteins in PXG, but its production is exhausted and its immunoreactivity in eye tissues diminishes. In other similar pathophysiologic situations such as drusen, corneal amyloid, arteriosclerotic plaques senile plaques in Alzheimer’s disease, prominent initial immunoreactivity for clusterin is present. Continued oxidative stress and mitochondrial lysosome axis dysfunction may exhaust clusterin levels and reduce clearance of misfolded protein and increase tissue fibrosis [56,57].
Despite the well-known higher prevalence of pseudoexfoliaiton glaucoma in Scandinavia, a US cohort of Scandinavian ancestry was not found to be at risk for pseudoexfoliaiton glaucoma [58]. Environmental factors, such as number of days of solar exposure were suggested to play a role there. Others have linked lifetime residential latitude from the equator with increased odds of pseudoexfoliaiton glaucoma [59].
The variable risk of pseudoexfoliaiton glaucoma with some LOXL1 polymorphisms but not with others, as well as protective LOXL1 alleles in some populations, suggests that these alleles in different environments or under different stress factors, and even in different systemic diseases, (which have been associated with pseudoexfoliaiton glaucoma) may behave differently [44,60,61].
Chronic diseases of the brain, such as cerebral atrophy, chronic cerebral ischemia, and dementia were more common in PXG than POAG [62]. While some studies have illustrated risk of carotid, renal and cardiovascular disease in PXG, [63-67], others have not found linkage to cerebrovascular and cardiovascular disease [62]. A metaanalysis, however suggested the association of pseudoexfoliaiton syndrome with coronary artery disease, cerebrovascular disease and aortic aneurysm [64]. Despite the variability of pseudoexfoliaiton glaucoma with different systemic diseases, it would appear albeit paradoxically that multiple studies have not shown increased mortality in patients with pseudoexfoliaiton, [63,66,67], and specifically a decrease in overall mortality and no difference in cardiovascular or cerebrovascular mortality [68]. Another study from Finland, interestingly, found increased life expectancy in pseudoexfoliaiton glaucoma patients compared to patients with POAG [69].
Poor pupillary dilation and loose zonules in pseudoexfoliaiton patients presents greater intraoperative complication risk with cataract surgery. Over half the late intraocular lens/capsule dislocations that occur more than three months after cataract surgery are related to pseudoexfoliaiton syndrome [70].
Corneas in patients with pseudoexfoliaiton regardless of the presence of glaucoma have decreased endothelial cell density and the central cornea is thin [71]. Decreased tear production and tear film stability has also been observed in pseudoexfoliaiton syndrome [70].
It is possible that some of the above findings in patients with pseudoexfoliaiton syndrome may relate to hypoxia, oxidative stress and ischemia. On electron microscopy, progressive iris vasculature degeneration from adventitia to endothelium was noted in the setting of hypoxia in pseudoexfoliaiton patients not seen in controls [72]. Subfoveal choroidal thinning was more recently noted in pseudoexfoliaiton eyes perhaps secondary to choroidal hypoperfusion secondary to impaired carotid flow [73,74]. Color Doppler imaging of the ophthalmic artery in pseudoexfoliaiton glaucoma patients identified increased vascular resistance [75]. Oxidative stress and inflammation have been cited in patients with early pseudoexfoliaiton syndrome. Elevated oxidative stress with decreased antioxidant capacity was found in AH of pseudoexfoliaiton patients compared to controls [76]. TNF-alpha, IL-6, and IL-17 were also reported increased in AH of early stage pseudoexfoliaiton glaucoma patients compared to controls [77].
Prominent Aqueous Humor Proteomic Changes in POAG, elevated:
1) immune globulin and alpha trypsin inhibitor (immune and inflammatory responses),
2) lipid metabolism (protein-lipid, lysosomal degradation transport, clearance),
3) ATP dependent chromatin remodelling (epigenetic energy dependent methylation/acetylation, balancing, re- balancing),
4) glycoproteins (associated with ECM function and remodelling)
5) isocitrate dehydrogenase (NAD mitochondrial balance)
In fact, in line with higher inflammatory and immune response in the AH of PXG patients, using liquid chromatography-mass spectrometry informatics, 33 distinct proteins from the aqueous humor of POAG glaucoma patients were identified. They were characterized by such diverse cell functions such as signalling, glycosylation, immune response, molecular transport and lipid metabolism [78]. An increasing trend in the odds ratios of having POAG was noted with increased levels of these proteins in the aqueous humor, AH. The four highest protein levels in AH proteins were found in Ig j chain C region:13.56 fold (immunity related), inter-a-trypsin inhibitor heavy chain 4: 4.10 fold (protease inhibitor, also involved in immunity and inflammation), apolipoprotein C III: 3.36 fold (triglyceride metabolism), and isocitrate dehydrogenase (NAD) (mitochondrial enzyme for energy balance) 3.1 fold [78].
To understand associations of the 33 significantly altered proteins in POAG, bioinformatics (canonical pathways using ingenuity pathway analysis) in POAG were compared to controls.
Thus, the proteins in the POAG group were highly involved in immune, inflammatory response, lipid metabolism, (likely lipid transport in and out of lysosomes), extracellular space activities (as in the aqueous humor), repressor element silencing transcription factor (REST), and ATP dependent chromatin remodeler transcription factor [78].
Fifteen of the thirty-three elevated AH proteins in POAG belong to the glycoprotein and glycosaminoglycan category. One of the key components of the POAG juxtacanalicular TM tissue is accumulation of ECM fibrillary elements associated with glycoproteins [78,79]. These TM proteomic distinctions will be reviewed in several of the later result topics such as myocilin/ misfolded protein, oxidative stress, and pigmentary dispersion syndrome and signal pathways in glaucoma.
MYOC TM Endoplasmic Reticulum Dynamics: Mutations/ Misfolded Proteins trigger Autophagy; Myocilin and IOP Increase after Oxidative Stress; overload misfolded protein results in amyloid myocilin accumulation and TM thickening and pre apoptotic signalling; secondary autophagic pathway activated through Unfolded Protein Response for misfolded protein overload; sustained elevated IOP (Intraocular Pressure) results in NFKB/ IL1 and variable Metalloproteinase, MMP, IOP responses, which can relax TM and lower IOP, or increase TIMP3, inhibit MMP and raise IOP; and Dexamethasone raises fibronectins, tightens ECM and raises IOP; Antioxidants, higher Temperatures: protective: less myocilin misfolding, lower IOP
MYOC gene, which codes for myocilin protein (highly expressed in the TM), is likely inherited as a familial trait, and may be helped by familial genetic assessment and counselling. It has been associated with a more aggressive, juvenile onset open angle glaucoma (accounting for 10%) and adult onset open angle glaucoma (accounting for 2-4%). Mutations in myoc-OLF, olfactomedin domain, (protein structured like five bladed beta-propellers, beta protein sheets around a central axis), are responsible for aberrant myocilin protein and enzyme interactions and thought to be associated with the development of glaucoma [80,81]. They have a sodium and calcium ion and glycerol molecule within a central hydrophilic cavity that is accessible after movements of surface loop residues [81].
That said, it is unclear if overexpression of mutant myocilin is the actual source of development of glaucoma. For example, Pro370Leu is one of the most severe glaucoma phenotypes, which is tied to TM cell mitochondrial dysfunction leading to apoptosis [80,82]. However, Gln368Stop, which is the most common myocilin mutation, has low penetrance for glaucoma. So, from the genetic standpoint, while mutant associations do exist, it is unclear whether various mutant allele types determine gain or loss of function with respect to the pathogenic glaucoma mechanism [80,82-85].
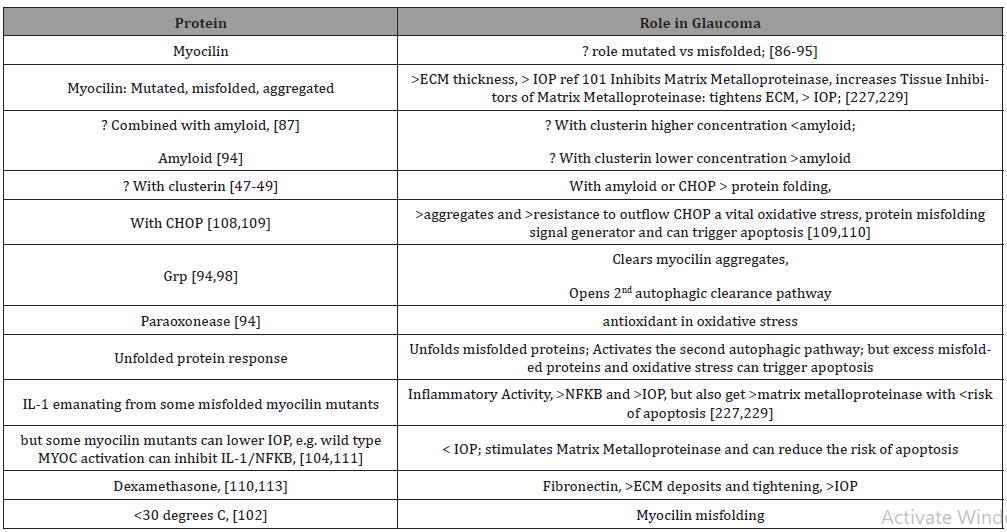
Recent studies [86-88] have indicated that the lack of sufficient myocilin is not the source of glaucoma, but that it is dependent on the over expression of mutant/misfolded myocilin. It is the aggregate of the misfolded proteins in the endoplasmic reticulum of the TM cells which produces stress in the endoplasmic reticulum as well as increased oxidative stress which leads to excess reactive oxygen species, reduction/oxidation imbalance, decreased antioxidative enzymes and to apoptosis [89,91-93]. Repeat misfolded protein accumulation without clearance of degraded products leads to amyloid containing myocilin [94]. This adds to the reduced clearance in glaucoma and with the greater protein aggregate raises IOP and further damages outflow channels [94].
Interestingly, co-aggregation of Grp94, glucose regulated protein 94, with mutant/misfolded myocilin OLF protein while resulting in greater endoplasmic reticulum deposits also enhances coprecipitation of myocilin-OLF [94,95,97] and has been suggested as an anti-glaucoma therapy to clear mutant/misfolded myocilin [94,95,97]. Alternatively, using a secondary autophagic pathway through 4-Br-BnIm (which is a Grp94 inhibitor), 4-Br-BnIm clears aggregated myocilin and alleviates myocilin TM toxicity [98]. Thus, with TM endoplasmic reticulum congestion from excess mutant misfolded myocilin aggregates, co-precipitators, like Grp94, as well as two autophagy pathways can help alleviate the toxic misfolded protein accumulation.
Apparently under normal conditions, myocilin misfolded aggregates are cleared by ubiquitin-proteasome lysosomal pathways. However, mutated or misfolded myocilin, after dysfunction of the primary proteasomal pathway, induce a secondary autophagic pathway (through activated unfolded protein response UPR) in an endeavour to protect TM cells. So, while Grp94 may act as a co-aggregator of myocilin misfolded protein and reduce their contribution to glaucoma, it may also induce the alternate clearance autophagy pathway. [95,96,99,100] Grp94 inhibitors prevent Grp94 from aggregating with myocilin mutants, but also result in initiation of the secondary autophagic pathway leading to mutant myocilin clearance [96].
The TM in patients with glaucoma with myocilin mutations is thicker than that of patients without myocilin mutations [101]. Misfolded myocilin aggregates in the ER of TM cells not only illustrate morphological changes but also lead to apoptosis [90,101,102]. The mutant misfolded myocilin aggregates in the ER promote increased reactive oxygen species, [89,95,96,102] induce TM ER dysfunction, apoptosis, and increased IOP [86-87,105-107]. Reactive oxygen species that are then in the aqueous humor plus those from the TM cause additional protein misfolding [93]. So, it becomes a vicious cycle of cellular and aqueous reactive oxygen species increasing mutant myocilin misfolding which renders cells more sensitive to oxidative stress [93-94]. Mutant myocilin inhibits antioxidant enzymes, such as paraoxonease 2, which with less oxidative stress efficiently inhibits endoplasmic stress-induced apoptosis [94]. Also, with greater oxidative stress, aggregated misfolded myocilin initiates the unfolded protein response, UPR, which (as mentioned above activates the alternate autophagy response to help protect TM cells), [107,108], reduce protein misfolding and degrades misfolded proteins [108-109]. If ER stress is not checked, then UPR induces apoptosis [89-90]. The aggregate myocilin misfolded proteins after high ER and oxidative stress aggregate amyloid to the myocilin misfolded products resulting in more severe glaucoma [89,94]. Thus, in addition, the excess aggregates are not readily cleared by the primary proteasomal pathway and require Grp94 inhibition with 4-Br-BnIm to reduce misfolded myocilin protein pile-up and TM toxicity [93,94,97] via the secondary autophagic pathway [95,99,100].
In line with the activation of IL-1/NFKB inflammatory stress response in high tension glaucoma [110], other proinflammatory mediators such as IL1 beta and mutant myocilin aggregate within the TM cells, activate the NFKB inflammatory pathway and raise IOP. Extracellular mutant myocilin, (outside the TM cells), does not activate NFKB signaling pathway [111].
The flip side to this is that IL-1 by stimulating matrix metalloproteinase MMP [111] and inhibiting apoptosis caused by oxidative stress through NFKB, [110] may reduce IOP. However, both IOP and oxidative stress are associated with the aggregation of misfolded mutant myocilins [86]. Interestingly, the aggregation of different misfolded mutant myocilins may upregulate IL-1 to different levels. Gln368Stop increased IL-1 tenfold in TM cells while those TM cells harboring Try437His induced IL-1 only sixfold. Gln368Stop, with the higher anti-inflammatory effect of IL- 1, had a greater IOP reduction than Try437His in the short term. Gln368Stop is the most prevalent mutation but exhibits less elevated IOP glaucoma phenotype [80,111].
Matrix metalloproteinase 2, MMP2, which is abundant in TM [112], is involved in extracellular matrix breakdown [113] and facilitates the outflow of aqueous humor. However, myocilin mutations, or MYOC null, can increase TIMP3, tissue inhibitor of metalloproteinase, which can inhibit MMP2 and cause POAG [114]. Lowering MMP2 can reduce the breakdown of extracellular matrix altering TM function leading to elevated IOP [80,93,116].
In addition to metalloproteins, inflammatory cytokines, misfolded/ mutant myocilin, amyloid, clusterin, reactive oxygen species, antioxidant enzymes, primary and secondary proteasome/ lysosome proteins (all interacting in the dynamic of the TM/ aqueous humor outflow system), fibronectin [115] and flotillin-1 [116] have also been identified as interacting with myocilin. The five bladed protein propellers of myocilin-OLF is an accepted bioenergetic platform for protein-protein interactions [80,81].
Mice with myocilin-Tyr437His which is less of an antiinflammatory inducer of IL-1, have a higher fibronectin and CHOP (a TM ER stress marker) protein expressions [115]. Mutant myocilins in another study [110] increased CHOP and Grp78 in TM.
Of note is that following dexamethasone anterior chamber treatment [117] myocilin, fibronectin, CHOP and Grp78 levels were increased. Treatment with sodium 4-phenylbutyrate decreased the dexamethasone induced increased IOP and fibronectin aggregation in TM cells [117]. The failure of the TM to control ER stress from misfolded/mutant myocilin results in increased CHOP which leads to apoptosis and increased IOP in TM cells [109].
Flotilin-1 a structural protein of the plasma membrane, which is rich in cholesterol and sphingolipids and involved in moving protein molecules from the cell surface to the interior, (like a raft), using endocytosis, also interacts with myocilin. Myocilin mutations, however, including Tyr437His and Gly364Val fail to interact with Flotilin-1 [117]. The absence of myocilin mutant interaction with flotillin-1, because of its centrality to aqueous humor protein dynamics may be another step-in protein mismanagement and glaucoma progression [117].
A severe myocilin glaucoma mutant, Pro370Leu, co-expresses hevin a protein involved in assembly of extracellular matrix [118]. The myocilin mutation causes intracellular accumulation of hevin and reduces its secretion compromising the production of extracellular matrix [119]. This may represent yet an additional step in the development of glaucoma, albeit not through the TGF beta pathway, but rather through cell adhesion to fibronectin or myocilin like binding through its homologous C-terminal domain to modulate ECM proteins [119].
Returning to the myoc-OLF protein structure, additional evidence has pointed to the relationship of pathogenic myocilin mutation aggregation and thermal instability [89,102]. The largest number of mutations occur in core beta-sheet belts (40%), loop B-10/C-11 and cation pi (33%). These misfolded mutants show a difference in melting temperature which is about two degrees C lower than wild type myocilin-OLF and resulted in more intracellular insoluble aggregates and early onset, more severe glaucoma [80,102,120-122]. Trimethyl N-oxide and sarcosine hold the number of myocilin melting temperature mutations to near the wild -type myocilin levels [123]. 30 degrees C is known to facilitate proper protein folding [122,124]. Thus, lower temperatures or temperature sensitivity (and possibly their greater oxidative stress/ energy deficient sources) may be another assailable area in proteomic misfolding and glaucoma development [80]. (Table 2)
Table 2:Short List of Myocilin Proteomics and Glaucoma.
Pigmentary Dispersion Syndrome (PDS)/Pigmentary Glaucoma (PG), differences from PXG, TM pigment deposits instead of punctate deposits of PXG; phagocytic pigment stress alters TM/ ECM clearance resulting in dysfunctional TM, adhesions, and cell death; premelanosome protein, essential for melanosome melanin, synthesis, storage and transport, had structural changes to pseudo melanosomes with altered amyloid fibril formation and significant ocular pigmentation defects; complex additional pigment protein interactions linked to melanin toxicity, immune attempts at clearance, mitochondrial dysfunction, and apoptotic cell progression; mechanical forces in PG
Pigment dispersion from the iris pigment epithelium and possibly retinal pigment epithelium [125] into the anterior segment produces iris transillumination defects in 86% of patients with PDS [126], dense pigment in the TM, abnormal iridozonular contacts [127,128] and pigment deposits on the back of the cornea, Krukenberg’s spindle [129,130]. The deposits on the back of the cornea are observed in about 90% of PDS patients but do not correlate with glaucoma/corneal thickness differences [131,132]. Unlike the patients with pseudoexfoliaiton syndrome who have punctate deposits of material in the TM, PDS patients have diffuse and uniformly dense pigmented TM [133]. Histologic exam showed that TM and corneal cells phagocytize the excess pigment granules but are then subject to phagocytic stress which compromises TM ECM structure and causes adhesions. TM cells become dysfunctional, exhibit local and then more extensive necrosis and death [134]. Aqueous humor outflow is reduced and PDS is converted to PG [135,136]. The amount of TM pigmentation corresponds to the severity of the optic neuropathy in PG patients [137,138]. However, the defect in retinal pigment epithelial cells degranulating is unclear.
PG represents about 1.25% of the glaucoma cases and since its early age of onset, it is the most common cause of non-traumatic glaucoma in young adults. Although not as yet replicated, analysis of four pedigrees of three generations, with Irish or mixed Western European ancestry affected by PDS/PG showed an autosomal dominant transmission, which mapped to human chromosome (7q35-q36) [138]. Other potential genes include, human endothelial nitric oxide synthase which maintains vascular tone and may contribute to iris structural defects [139-141]. MYOC and lysyl oxidase genes have had suggested limited association with PDS/ PG glaucoma, but because of small number of associated variants of the former and small sample size of the latter [142-147] causal relationship is lacking. Known anterior segment developmental control genes such as PAX6, [148], FOXC1 [149] and PITX2 [150] are not associated with PDS/PG but rather with anterior segment dysgenesis, FOXC1 and PITX2, and with microphthalmia, PAX6, [151].
Missense mutations in Tryp1, alleles (which would otherwise produce tyrosinase and normal melanin pigment) has produced iris atrophy in mouse strains. Alteration of cysteine residues in Tryp1 alleles also causes the release of toxic melanin intermediates from melanosomes, which result in melanocyte death. Yet other genetic mouse mutations, such as in Dct, has also been associated with PDS through the tyrosinase complex and may produce melanosome dysfunction and cytotoxic melanin synthesis intermediates [152- 156]. The human homologue OCA2, a melanosome regulator of pH, has been linked to oculocutaneous albinism type 2, and has been associated with iris color [157-161].
Some recent work suggested a strong possible protein driver of PG, defective processing of PMEL, (premelanosome protein identified in humans with PDS/PS using whole exome sequencing of the two independent pedigrees). PMEL, essential for melanosome melanin, synthesis, storage and transport, had structural changes to pseudo melanosomes with altered amyloid fibril formation in five of nine gene variants. CRISPR introduction of the 11-base pair deletions to homologous PMEL in zebrafish caused significant ocular pigmentation defects and enlarged anterior segments. This suggested that variants in human PMEL are responsible for PDS/ PG [163].
DBA/2 mice, which have iris transillumination defects [161, 162], have other gene defects that have been implicated in pigment dispersion phenotypes, some of which tie into immunosurveillance, trafficking, and melanocytic detoxification. Gpnumb, (glycoprotein nonmetastatic melanoma protein) which is involved in containing melanosome cytotoxic melanin synthesis intermediates, has additional neuronal and immune cell adhesion functions. Lyst is another protein traffic monitor but at the lysosome interface for early melanosome variants. The latter can cause Chediak-Higashi syndrome, (childhood immune disorder with reduced skin and ocular pigment, immune deficiency with frequent infections and easy bruising and bleeding). However, iris pigment epithelial dysfunction, whether mediated by: (1) release of synthesis, transport, or storage inept premelanosome derivatives, cytotoxic melanosome derivatives, (2) oxidative stress with excess melanogenesis over and above routine iris pigment maintenance, (3) inappropriate immune monitoring and clearance of excess melanin variants (3) cytotoxic melanin pigment metabolic derivatives, (4) inappropriate melanosome/mitochondrial interfacing and trafficking, and (5) melanosome apoptosis or necrosis from inflammatory mediators [164-168] has left these oxidative stress/metabolic/inflammation and immune research topics open for further investigation.
In the realm of linking signal transduction pathways, Myo5A for myosin, protein kinase C, PKC, for signaling, and transcription factor zinc finger ZLbtb20 have been associated with iris transillumination defects [162]. The association of Myo5a and protein kinase c has been through intercellular melanocyte movement [165-167] or melanocyte dendrite formation [167]. Interestingly, suppression of Rho A, a target gene of micro RNA340 and promoter of actin, appears to regulate UVB induced dendrite formation and melanosome transport as well [169].
DBA/2J mice develop a pigment dispersing iris disease that leads to elevated IOP and RGC loss. The mutant alleles of two genes, Gpnumb and Tryp1, respectively cause two components of iris disease, iris pigment dispersion and iris stromal atrophy [170]. The phenotype for iris pigment dispersion is similar to the pigment dispersion syndrome seen in humans [170,171]. By adding bone marrow of DBA/2J-Gpnmb+ mice to DBA/2J-Gpnmb- mice, the iris disease and subsequent IOP rise can be mitigated [172,173]. Bone marrow derived lineages of standard DBA/J2 mice may have different immune inflammatory responses than the DBA/J2+ mice. While adaptive T and B cell mediated components have been ruled out [172] other inflammatory immune or innate genetic immune responses may be at work [172,174].
On the other hand, if the structural iris concavity, prevalence of myopia, and excess iridozonular contact which removes pigmented cells from the iris pigment epithelium via mechanical rubbing in patients is the etiology, then midperipheral and radial iris transillumination defects may correlate to just mechanical forces [128,175-178].
That said, if the mechanical theory is a root cause of the glaucoma, (and there is a type of reverse pupillary block with iris concavity and subsequent abnormal iridozonular rubbing with pigment shedding), one might argue that laser peripheral iridotomy which would flatten the iris and avoid the chafing would retard the glaucoma progression. However, a large database review of laser peripheral iridotomy did not report any reduction in visual field loss or glaucoma progression [179-181].
Thus, the exact sequence of PDS phenotype pathophysiology is not yet clear and the genetic and proteomic markers from the models also need better correlation.
POAG genes: lipid, cholesterol levels: risk factors; Mitochondrial support gene regulation; Retinal Oxidative Stress, Misfolded Proteins, Mitochondrial/ Lysosome Pathway Clearance/ Dysfunction, Retinal Heat Shock Proteins/Antibodies, autoantibodies (in Glaucoma); Excess Inflammation and Immune Reaction, Apoptosis
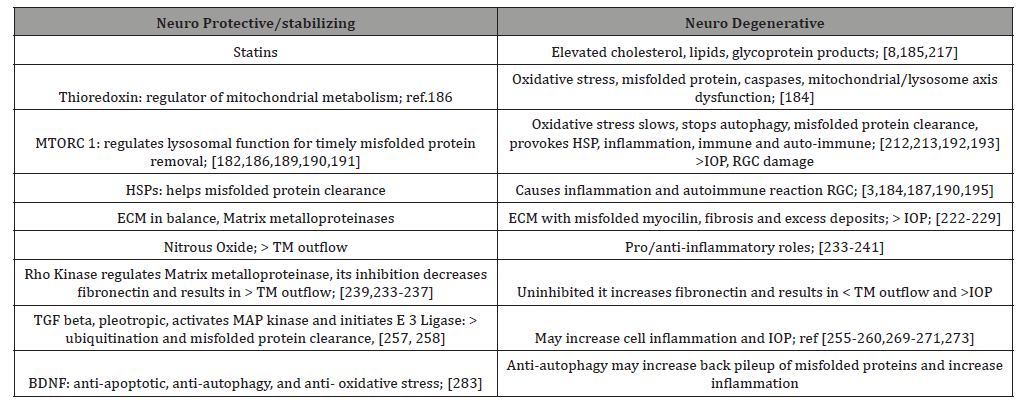
Oxidative stress related to different levels of cholesterol, lipid [8], glycoprotein products as well as mutations in genes for dysfunctional protein removal lead to endoplasmic reticulum protein misfolding, inadequate mitochondrial energy production, lysosomal dysfunction and toxic intracellular accumulation [182].
Genes ABCA1, CAV1, DGKG, and ARHGEF12, which are POAG and intraocular pressure loci, are involved in lipid metabolism [183]. High serum cholesterol has been associated with the risk of POAG, while statin use may be protective [184,185].
In a similar fashion, but different metabolic side of the picture, TXNRD2, thioredoxin 2, which regulates mitochondrial metabolism, and helps reduce oxidative stress, (as well as four other mitochondrial loci) have been associated both with POAG and IOP loci [186]. These lipid and primary metabolic/energy linkages may portend future approaches to glaucoma [8].
Elevated auto antibody levels against heat shock proteins (HSP) 27 and 60 were found in human donor retinae from normal and high-pressure glaucoma patients [187]. The heat shock proteins are highly conserved and ubiquitously expressed in response to metabolic, oxidative and thermal stress. They endeavor to prevent protein denaturation and aggregation and assist in refolding misfolded protein [188]. Immunogenic peptides either extracellularly or intracellularly and bound by the HSP are taken into lysosomes or proteasomes for degradation [189,190]. Thus, the effects of heavy oxidative stress result in dysfunctional mitochondrial proteins and other misfolded proteins which invoke the assistance of HSP which endeavor to help re-fold misfolded proteins and provide anti-apoptotic support as they arrange misfolded protein clearance either through the lysosome apparatus or immune aggregation [7,189,190].
Lysosomes fuse with autophagosomes to deliver the hydrolases that degrade the misfolded protein. mTORC 1 signaling regulates lysosomal function. Under nutrient rich conditions, mTORC1 phosphorylates transcription factors like MITF, Microphthalmiaassociated transcription factor, a master gene regulator for melanocytes, cell cycle progression, and differentiation. MITF then initiates timely cytosolic chaperone maintenance which inhibits autophagy misfolded protein clearance. In the absence of nutrients or under stress, autophagy inhibition is relaxed leading to lysosomal and autophagy gene induction [189,190]. Thus, the HSP are facilitators to misfolded protein degradation as well as to immune antibody reactions, protective and autoimmune [7].
Some of these protein antigens, however, are transported to the endoplasmic reticulum where they are loaded to the major histocompatibility complex, MHC, and transported to the cell membrane to present to the T cell as either foreign or endogenous which then leads to protective immune reaction [191,192]. However, in addition to the antigens triggering antibody reactions, some HSP proteins themselves trigger autoantibodies which can result in damage [7,193,194]. In rheumatoid arthritis patients, for example, cross reactive T cells have produced autoantibodies to self HSP as well as other antigens. This two-sided immune exuberance produces an inflammatory reaction, which may or may not be balanced by immunosuppressive activity of other HSP or yet other anti-inflammatory pathways [194,195-197]. Trauma, excess heat, chemical attacks, radiation, hypoxia and oxidative stress may incite or contribute to the HSP immune or inflammatory response. The plasticity of HSP-mediated immunity (i.e. priming T cells for various HSP mediated immunity, cytokine Th2, Th2 and Th 17, and/or T cell regulatory by itself) varies with the ability of HSPs to engage local antigen presenting cells, through cell surface receptors, thereby stimulating diverse costimulatory signals [194].
Wax and his group 20 years ago showed higher antibody levels against HSPs (alpha and alpha B crystallin, HSP27 and HSP60) in normotensive and high-pressure glaucoma groups than controls. Similar elevated autoantibodies to HSP in normotensive and highpressure glaucoma patients vs controls were made in U.S and Japanese populations [198-200].
In a rat model, intraperitoneal HSP immunization produced IOP related retinal ganglion cell density reduction after 28 days [201]. In later rat model studies, intravitreal HSP application also led to an IOP independent loss of retinal ganglion and amacrine cells after 21 days [202]. Others, [204,205] have demonstrated immunolocalization of HSP in normal and laser induced glaucoma monkey eyes as well as rat retinal ganglion and glial cells in rat glaucoma.
However, the exact role of HSP in glaucoma is unclear and may be situationally dependent [7]. As cytoplasmic chaperones, HSPs contribute to refolding of misfolded and denatured proteins. HSP27, HSP70 and the small heat shock proteins are known regulators of apoptosis, [205,206] stress induced apoptosis, [207], bind pro-apoptotic protein activators, [208], oxidative stress neuro-protectors in RPE environment, [209], inhibit autoproteolytic maturation, caspase 3 activation, [210], and axonal degeneration [211]. On the flip side, because of their role in misfolded protein, degenerative protein aggregates, ubiquitination, antibody generating capacity as well as autoantibodies and proinflammatory T cells and cytokines, HSP can cause inflammation and autoimmune degeneration [211-217].
POAG TM oxidative stress: (unable to sustain AH outflow); HSP, Juxtacanalicular resistance, ECM products and thickening; Endothelial cell/ mitochondrial energy demand, damage to mitochondria and ubiquitin pathway for damaged protein removal to proteasomes; greater oxidative stress and inflammation, apoptosis; progressive visual field changes with oxidative stress
Oxidative stress by heat shock (44 degrees C for 15 minutes or hydrogen peroxide, 200 micromole for 1 hour) to human and monkey fresh and cultured trabecular meshwork TM resulted in significant increase in alpha beta-crystallin HSP with a peak after four hours expressed predominantly in the cribriform area (juxtacanalicular, JCT, region near Schlemm’s Canal), the area of greatest resistance to aqueous humor AH outflow [218].
Over a longer time period due to imbalance between AH production and outflow [219-220] the main risk factor in POAG progression, the TM undergoes chronic inflammation and tissue remodelling [221,222]. Protracted oxidative stress produces TM and endothelial cell mitochondrial damage leading to neural degeneration and apoptosis [223,224]. The ECM develops increased collagen, fibronectin, and elastin with unusual myofibroblast development with thickening of trabecular sheets [221,225-229].
The AH of 14 patients with POAG, and not in controls, expressed elevated protein levels of ELAM, (endothelial leukocyte cell adhesion molecule expressed only on endothelial cells activated by cytokines, involved in inflammation), apolipoprotein B and E (for cholesterol delivery to cells.), three heat shock proteins, five muscle related proteins, a ubiquitin (for removal of stress damaged proteins), and three signal transduction proteins. These proteins may play a role as biomarkers for POAG [230] and form the core of protein mechanics in evolving glaucoma.
In later work, to better understand the TM ECM metabolism, fibrosis, and angiogenesis in 40 glaucoma patients, these investigators examined digital spectrophotometry/chip-based array AH protein profile expression vs controls. The inflammatory cytokines, particularly IL10, 6, 5, and 7 were all over tenfold increased. TNF alpha (a pro-inflammatory cytokine) was 2.48- fold increased; TNFR was increased over four-fold. MMP2 (matrix metallopeptidase 2, produced in cells throughout the body and becomes part of the extracellular matrix, which is an intricate lattice of proteins and other molecules that forms in the spaces between cells) was 3.18 increased; VCAM1, (vascular adhesion protein- a cell adhesion molecule), MIP (macrophage inflammatory protein), 1delta, and MIP1alpha were all increased greater than fivefold. Thus, the large anti-inflammatory response levels and the moderately elevated metalloproteinase level suggest steady profibrosis increment under the umbrella of heavy anti-inflammatory cover. The POAG ECM change over time may be excessive and/or aberrant and necessitate an anti-inflammatory arsenal of a large magnitude to maintain a consistent aqueous outflow, in the face of incremental resistance [231].
In 202 Japanese NTG and POAG patients, blood analysis was performed for oxidative stress levels and compared to controls. Lipid peroxides, ferric reducing activity and thiol antioxidant activity were measured using a free radical analyzer and statistically compared with mean deviation of visual field. Univariate and multivariate analysis suggested a positive correlation between mean visual field deviation and biologic antioxidant potential. Use of more medication for glaucoma and POAG (vs NTG) were also associated with worse visual field progression [232].
TM, JCT, ECM in normal, glaucoma, glucocorticoids
In normal human eyes, using specific antibody immune electron microscopic determination, basement membrane like material in the JCT was identified as mostly collagen type IV, laminin and fibronectin. Elastin, however, was found in the central area of sheath related plaques. Fibronectin, fibrillin-1, MAGP-1, (microfibril associated glycoprotein), decorin (a collagen protein found in human skin) and type VI collagen were seen in clusters of the banded material in the sheath surrounding the core. Myocilin associated mainly with sheath material such as fibronectin and fibrillin [233-237].
In patients with POAG, excessive abnormal accumulation of sheath derived plaques has been shown on EM, immune and histologic studies. Of particular interest is the association of myocilin with microfibrils, which may have a role in the evolution of sheath density, plaque development and aqueous outflow reduction, (see figure 5 in ref 233 paper) [233].
However, glaucomatous TM characteristically show increased rigidity and stiffness with the formation of cross-linked actin networks with greater aqueous outflow resistance.
An increase in fibrillar content in JCT ECM is prominent in POAG and steroid induced glaucoma [238]. Other studies have detected increased calcification of the TM, which may increase TM stiffness and increase outflow resistance in glaucomatous tissue [239].
Nitrous Oxide Donating Moieties for TM increased AH outflow
Therapeutic approaches to lowering IOP include: (1) reduction of aqueous production, (beta blockers, carbonic anhydrase inhibitors, and alpha agonists); (2) increasing uveoscleral outflow, (extracellular matrix remodeling, prostaglandin agonists); (3) contracting the ciliary muscle, (opening spaces in the TM), pilocarpine); (4), increasing trabecular outflow, nitric oxide, (NO donating moiety).
Nitrous oxide plays many roles in the body. It has anti-platelet function in type 2 diabetics, assists smooth muscle relaxation (through vasodilation) in cardiovascular, urogenital, gastrointestinal and respiratory settings, as well as pro/anti-inflammatory and pro/ anti-microbial roles in high/ low NO venues.
iNOS isoforms of NO (from inducible NO genes) is the predominant form in TM and eNOS (endothelial) is the major isoform expressed by SC cells [240-248]. NCX434, a NO donating triamcinolone acetate has been used to improve retinal vascular blood flow and improve optic nerve oxygenation in the cynomolgus monkey model of glaucoma [249,250].
Latanoprostene bunod, latanoprost combined with a NO donating moiety has been effective in lowering IOP even in the early morning hours when the IOP spike is common and affects the TM and SC to achieve additional IOP reduction vs. latanoprost alone, and was more effective by 1-2 mm Hg than both latanoprost and timolol over a 52-week period [251-253].
By inhibiting Rho kinase, a protein kinase which helps modify cell shape, size and adhesion by altering actin cytoskeleton, TM cells allow increased outflow of AH.
Netarsudil, the active compound in Rhopressa, FDA approved in 2017, uses rho kinase inhibitor which increases aqueous outflow and a norepinephrine transport inhibitor which by constriction of vessels to the eye reduces blood flow to the ciliary processes and aqueous production as well [254].
Signal Pathways in Glaucoma, TM: TGF beta, Rho Kinase, ECM contractile morphology, rigidity, MAP Kinase, E3ubiquitin ligase protein clearance; BDNF neuroprotection Signal Pathways in Glaucoma
In a normal setting, if threats of oxidative stress, hypoxia, trauma, mechanical forces result in inflammation and immune reaction, then transcription factors, (powerful nuclear genes) and histone modification of other protective genes establish defense plans, that are responsible for an efficient measured response. In glaucoma, the overactive TGF-beta signal pathway, which is usually helpful in healing, produces excess extracellular matrix protein deposition in the TM and blocks outflow of AH. Treatment of human TM cells with TGF-beta 2 upregulates Plasminogen activator inhibitor and secretion of fibronectin which increases extracellular matrix production. [7,256] TGF-beta2 induced ECM deposition and reduced outflow facility of aqueous humor by 27% in cultured human anterior segments [257].
TGF-beta2 is responsible for signaling differentiation, proliferation, chemotaxis and fibrosis. In healthy eyes TGF-beta2 helps corneal healing. In glaucoma TGF-beta2 causes increased extracellular matrix proteins in the TM cells resulting in fibrosis and blocked outflow of AH. Glaucoma patients have increased levels of TGF-beta2 in AH compared with normal [258,259].
TGF-beta increases ECM through the traditional Smad pathway (which conveys cell surface signals to intracellular mediators known as Smads, serine/threonine kinase receptors, causing their transfer from the cytoplasm to the nucleus, where they function to control gene expression), as well as the Mitogen-Activated Protein (MAP) kinase and Rho kinase pathways [7]. MAP kinase pathway leads to MAP ERK kinase resulting in increased ECM production in TM cells [255,258] and proinflammatory cytokine Interleukin 6 and Secreted Protein Acidic Rich in cysteine, SPARC, in TM cells. The latter binds to ECM proteins and regulate matrix metalloproteinase expression. In addition, p38 MAP kinase is activated when TFGbeta binds to its I and II receptors, which initiates an E3 ubiquitin ligase (a commanding officer for protein pick up and removal). This accounts for inflammation, ubiquitination, and ECM TM cell balancing and re-balancing in AH dynamics [251-261].
Rho Kinase, (which is involved in cell migration, proliferation), is involved in actin cytoskeleton changes. CLAN, cross-linked actin network, has been observed in TM cells of eyes with glaucoma. [264] While the exact method of CLAN “tightening” the activity of TM cells is unclear, the TM cells express elevated levels of: laminin, alpha-smooth muscle actin, matrix assembly, actin stress fibers and myosin light-chain phosphorylation all of which are associated with ECM [7,264]. The latter TM factors were linked to increased contractile morphology. Rho Kinase inhibitors decrease fibronectin and alpha-smooth muscle actin [265]. Moreover, the Rho Kinase inhibitors reduce cell rigidity and increase aqueous outflow and reduce IOP by 30% after 3 hours in rabbit eyes [265].
Looking at the RGC/optic nerve side of glaucoma, several factors: (1) BDNF, brain derived neurotrophic factor and (2) JNK, (jun n terminal kinase), both neuroprotectant and pro-apoptotic, (3) phosphoinositide -3 kinase, (PI-3 kinase), a neuroprotectant, (4) PTEN, phosphatase and tensin homologue, linked to axon degeneration, (5) Bcl-2, an anti-apoptotic, (6) caspases, preapoptotic, and (7) calcium calpain pathway an apoptotic inducer, interact with one another and yet other proteins in an attempt to balance and re-balance cellular stress factors like elevated IOP with neuroprotective elements vs apoptotic neurodegenerative responses [267-281].
For example, BDNF is produced in retina and brain and is essential for RGC survival [260]. In glaucoma with increased IOP, reduced BDNF transport to the optic nerve results in caspase activation and apoptosis. However, intravitreal injection of neurotrophic factor in rats after optic nerve injury was neuro protective [275,276] Table 3.
Table 3:Short List: Neuro Protective/Stabilizing vs Neuro Degenerative Elements in Glaucoma.
After cellular stress, such as uv radiation, heat shock, withdrawal of neurotrophic factors, or optic nerve transection, Jun N-Terminal Kinases, JNKs, pro-apoptotic pathway is activated. It is unclear if this pathway is purely apoptotic. Some studies have shown c- Jun activation may help RGC survival [277,278].
Epigenetics and Glaucoma
Hypoxia, oxidative stress and inflammation in the eye result in activation of protective transcription factors such as hypoxia inducible factor 1-alpha, Nrf2, (the major antioxidant transcription factor), and NFKB, (the major anti-inflammatory, immune transcription factor) which recruit enzymes that either open up protective gene activity, such as histone acetylase, or repress gene activity (histone deacetylase or histone methylation modifiers) by acting on the histones which enshroud the genes. The histones affect the expression of the genes without mutations, by inducing amongst other signal devices, noncoding RNA, MicroRNAs and long noncoding RNAs. These signals promote certain rescue pathways or in some cases, with over stressed systems lead to greater disease susceptibility by blocking helpful pathways. For example, the introduction of MicroRNA-483-3p to stressed human trabecular meshwork cells decreased ECM production and reduced fibrosis and enhanced aqueous outflow facility [286]. However in another gene signaling, MicroRNA 204 by decreasing FOXC1 as well as its target genes Clock, Plekshg5, ITG beta 1, Meis2 may cause Axenfeld Rieger Syndrome, (a childhood glaucoma with iridocorneal adhesions, ectropion uvea, atrophic iris holes, with possible systemic associations, thought to result from abnormal neural crest cell migration [6].
Comparing HSP70 expression levels in human lens capsule of pseudoexfoliaiton syndrome, PXS, with those who had pseudoexfoliaiton glaucoma, investigators recently demonstrated that HSP70 levels were significantly reduced in the PXS vs PXG compared to controls. Bisulfite sequencing of the of the transcription pre-coding and coding sites showed hypermethylation of the precoding sites only in the PXS individuals. There was also a reduced corresponding increase in DNA methyltransferase 3A only in the PXS individuals [293]. This implied a timely methylation at the PXS stage of the disease. In contrast, peripheral blood of PXS and PXG patients showed hypermethylation in the transcription coding region when compared to non-PXS controls. This would suggest a local tissue specific effect of the HSP70 chaperone [293].
Further analysis of the DNA spanning the precoding and coding region illustrated that there was decreased gene expression in the methylated vs the un-methylated reported gene vectors. DNA methyltransferase inhibitor used to treat the human lens epithelial cells restored the expression of HSP70 which correlated with methylation reduction at the precoding sites [293].
These findings not only increase our knowledge of HSP70 reduction in conjunction with hypermethylation, an established epigenetic mechanism for reduced genetic expression, but may also provide a platform for regulating the early phase of pseudoexfoliaiton glaucoma.
Discussion
One can infer from the multitude of neuro-degenerative vs neuro-protective factors that a rather complex series of checks and balances may be operational in the RGCs, the optic nerve and the TM over the course of time. That said, while IOP control and the dynamics of TM outflow and the flexibility of the ECM to deposits and contractile mechanics are major factors in current glaucoma therapeutics, RGC, optic nerve and brain [287] homeostasis, its mitochondrial energy producing and sustaining capabilities, the ability to clear misfolded protein, and exert antioxidant, anti-inflammatory, anti-autoimmune, and anti-apoptotis signal pathways also constitute a major neuro-protective defense arsenal that will probably guide the glaucoma therapeutics of the future.
Thus far, targeting the complexity and numbers of neuroprotective agents seems to be a daunting task. Over 100 neuro-protective drug candidates have failed to demonstrate patient benefit. For example, Memantine, a N-methyl-D-aspartate, NMDA subtype of glutamate receptor antagonist already in use for Alzheimer’s disease, while convincing in animal models, five years and $100 million dollars later, failed to halt progression of visual field loss in glaucoma patients [287].
Also, animal studies which rely on histopathologic endpoints to examine the effectiveness of treatment are not practical in humans for ethical reasons. Perhaps newer methods of following RGC damage and demise such as: detecting apoptosing retinal ganglion cell technique, DARC, in vivo or methods of measuring mitochondrial depolarization, excessive glutamate receptor activation resulting in excess PTP, mitochondrial permeability transition pore, and excess of cytochrome c formation, (a preapoptotic), or overactivation of NMDA and non-NMDA glutamate receptors with resulting excess NO, refined VEP, visual evoked potential and PERG, pattern electroretinogram will serve as better guides in developing neuroprotectants [287].
Early data in CoQ10, (a component of mitochondrial respiratory chain by which ATP is produced) may be promising. Because of its role in protecting lipids and DNA from oxidative stress, it may inhibit mitochondrial pore formation and cytochrome release leading to apoptosis [288,289].
Citicoline, a neuro protectant used in Alzheimer’s Disease possibly interfering with the deposition of neurotoxic proteins such as beta amyloid [290] (and associated with improvement of rigidity, bradykinesia and tremor in Parkinson’s), has shown antiapoptotic effects in RGC mitochondria-dependent cell death [291]. Rats after nerve crush injury had a citicoline anti-apoptotic effect as well as an expression of Bcl-2, which is one of the main anti-apoptotic proteins [292].
Conclusion
Genomic, proteomic, epigenetic and molecular signaling pathways in the development of glaucoma represent the language of the next frontier to be conquered in human disease. Understanding the fine details of these pathways will provide a way to differentiate phenotypic/genotypic variances as well as better lines of therapeutic intervention.
Insights into the balancing and re-balancing of: (1) oxidative stress, (2) misfolded proteins, (3) heat shock proteins (including their immune-autoimmune/inflammatory and microglial effects), (4) key transcription factors, signal transducers, and chromatin gene modifiers, (5) mitochondrial/ lysosome protein misfolded clearance pathway, immune and proteolytic, and (6) the control of the caspases to prevent apoptosis may open directives for treatment of the non IOP part of glaucoma and neurodegenerative disease.
To read more about this article...Open access Journal of Ophthalmology & Vision Research
Please follow the URL to access more information about this article
To know more about our Journals...Iris Publishers
To know about Open Access Publishers





No comments:
Post a Comment