Authored by Ricardo Gobato*,
Abstract
The core of the work is based on the replacement of carbon atoms by silicon atoms, on the basis of four standard bases of DNA: A, C, G and T (adenine, cytosine, guanine, thymine). Determining with minimum computational methods via ab initio Hartree-Fock methods, infrared spectrum and their peak absorbance frequencies. The option for simple replacement of carbon by silicon is due to the peculiar characteristics between both. Atomic interactions under non-carbon conditions were studied, with only the Hydrogen, Silicon, Nitrogen and Oxygen atoms, in CNTP, for the four standard bases of DNA, A, C, G and T, thus obtaining by quantum chemistry four new compounds, named here as: ASi, CSi, GSi and TSi. Computational calculations admit the possibility of the formation of such molecules, their existence being possible via quantum chemistry. Calculations obtained in the ab initio Unrestricted and Restrict Hartree-Fock method, (UHF and RHF) in the set of bases used Effective core potential (ECP) minimal basis, UHF CEP-31G (ECP split valance) and UHF CEP-121G (ECP triple-split basis), CC-pVTZ (Correlation-consistent valence-only basis sets triple-zeta) and 6-311G**(3df, 3pd) (Gaussian functions quadruple-zeta basis sets).
Keywords:DNA; Adenine; Cytosine; Guanine; Thymine; Hartree-Fock method; Nano-molecule; Infrared spectroscopy; CEP-31G; CEP-121G; CCpVTZ, 6-311G** (3df, 3pd)
Introduction
The Deoxyribonucleic acid (DNA) is a molecule composed of two polynucleotide chains that coil around each other to form a double helix carrying genetic instructions for the development, functioning, growth and reproduction of all known organisms and many viruses. The A, C, G, and T, - adenine, cytosine, guanine and thymine, respectively, representing the four nucleotide bases of a DNA strand – adenine, cytosine, guanine, thymine – covalently linked to a phosphodiester backbone [1].
The core of the work is based on the replacement of carbon atoms by silicon atoms, on the basis of four standard bases of DNA: A, C, G and T (adenine, cytosine, guanine, thymine). Determining with minimum computational methods via ab initio Hartree-Fock methods, infrared spectrum and their peak absorbance frequencies [2-14].
The option for simple replacement of carbon by silicon [15,16] is due to the peculiar characteristics between both. Atomic interactions under non-carbon conditions were studied, with only the Hydrogen, Silicon, Nitrogen and Oxygen atoms, in STP (Standard Temperature and Pressure), for the four standard bases of DNA, A, C, G and T, thus obtaining by quantum chemistry four new compounds, named here as: ASi, CSi, GSi and TSi. In comparing the carbon and silicon has: the Si lies in the same column of the periodic table of the elements, and it has been investigated as a possible alternative for building up biological molecules in exobiology [17,18]. Silicon based chemistry, however, is by far less flexible than carbon chemistry, not able to form double covalent bonds with the same easiness as C does. Other fact is the larger volume occupied by the external electronic orbitals of silicon tend to reduce the superposition of p orbitals [17,18]. Through the chemical abundances of biolog ical elements in the earth crust, terrestrial life has chosen carbon instead of silicon, in spite of the larger abundance of silicon. This fact suggests that carbon is better suited to form biological molecules [17,18].
However, this paper assumes conditions without the presence of Carbon. Calculations obtained in the ab initio Unrestricted and Restrict Hartree-Fock method, (UHF and RHF). The set of basis used Effective core potential (ECP) minimal basis, UHF CEP-31G (ECP split valance) and UHF CEP-121G (ECP triple-split basis), CCpVTZ (Correlation-consistent valence-only basis sets triple-zeta) and 6-311G**(3df, 3pd) (Gaussian functions quadruple-zeta basis sets) [2-14]. Studies did not reveal any works with characteristics studied here. There is an absence of a referential of the theme, finding only one work in (Gobato et al., 2018-2021) [19-30].
Methods
Hartree-Fock Methods
The molecular Hartree-Fock [2-14] wave function is written as an antisymmetrized product (Slater determinant) of spin-orbitals, each spin-orbital being a product of a spatial orbital ϕi and a spin function (either α or β).
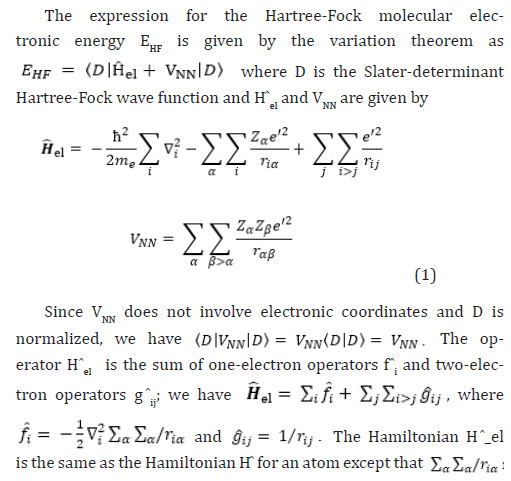
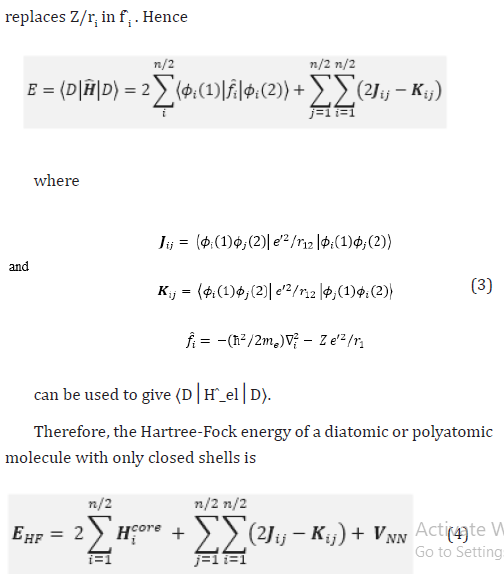
Therefore, the Hartree-Fock energy of a diatomic or polyatomic molecule with only closed shells is
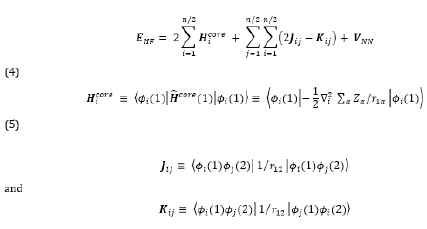
where the one-electron-operator symbol was changed from f ̂i to H ̂core (1) [4].
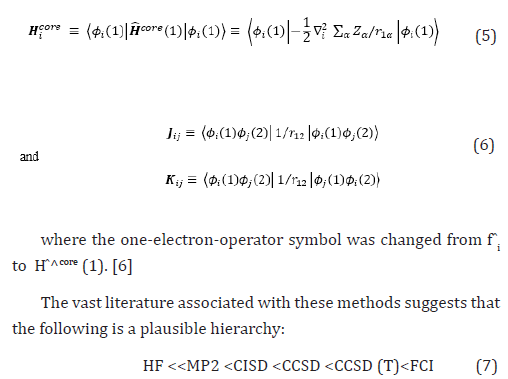
The vast literature associated with these methods suggests that the following is a plausible hierarchy:
HF <
The extremes of ‘best’, FCI, and ‘worst’, HF, are irrefutable, but the intermediate methods are less clear and depend on the type of chemical problem being addressed. The use of HF [2-14] in the case of FCI was due to the computational cost.
Hardware and Software
For calculations the computer used for MD (molecular Dynamics) was a Desktop with SUSE Linux Enterprise Desktop, AMD Ryzen 7 1800X processor, ASUS Prime A320M-K motherboard, 16GB of RAM, with 500GB SSD [31].
The ab initio calculations have been performed to study the equilibrium configuration of ASi, CSi, GSi and TSi molecules. The set of programs GaussView 5.0.8 [32], Mercury 3.8 [33], Hyper- Chem 8.0.6 Evaluation [34]. are the advanced semantic chemical editor, visualization, and analysis platform and GAMESS [15,35] is a computational chemistry software program and stands for General Atomic and Molecular Electronic Structure System [15,35], Origin- Lab 2018 Evaluation [36], BIOVIA Draw 2017 [37], CHARMM22 [38] set of programs were used.
Result
The molecular structure of ASi, CSi, GSi and TSi molecules, were obtained through computationally calculated molecular dynamics, using the ab initio Hartree-Fock (HF) method.
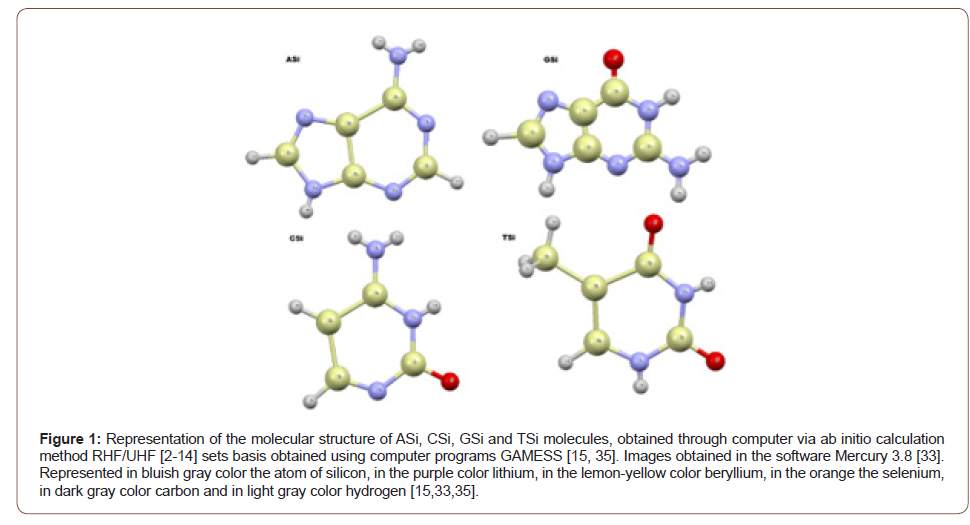
The names in Figure (1) of the new molecules obtained ASi, CSi, GSi and TSi, are:
2,3,4,5,6,7,8,9-octahydro-1H-[1,3,2,4,5] diazatrisilolo[4,5-d] [1,3,2,4,5,6]diazatetrasilin-8-amine;
2-hydroxy-1,3,2,4,5,6-diazatetrasilinan-4- amine;
8-oxo-3,7-dihydro-[1,3,2,4,5] diazatrisilolo[4,5-d][1,3,2,4,5,6] diazatetrasilin-6-amine and
(2,4-dihydroxy-1,3,2,4,5,6-diazatetrasilinan-5-yl) silane, respectively (Figure 1).
ASi
Properties of molecule Asi:
IUPAC name:
• 2,3,4,5,6,7,8,9-octahydro-1H-[1,3,2,4,5] diazatrisilolo [4,5-d] [1,3,2,4,5,6]diazatetrasilin-8-amine;
• PSA(Polar Surface Area): 74.14;
• ALogP: 7.1232;
• Stereo Center Count: 3;
• Hydrogen Acceptor Count: 5;
• Hydrogen Donor Count: 5;
• Composition: H: 5.9% N: 31.3% Si: 62.8%;
• Formula Weight: 223.56421;
• Exact Mass: 223.001728116;
• Molecular Formula: H13N5Si5 [37].
CSi
Properties of molecule CSi:
IUPAC name:
• 2-hydroxy-1,3,2,4,5,6-diazatetrasilinan-4-amine;
• PSA: 70.31;
• ALogP: 6.022;
• Stereo Center Count: 2;
• Hydrogen Acceptor Count: 4;
• Hydrogen Donor Count: 4;
• Composition: H: 6.1% N: 23.2% O: 8.8% Si: 61.9%;
• Formula Weight: 181.44883;
• Exact Mass: 180.997918122;
• Molecular Formula: H11N3OSi4 [37].
GSi
Properties of molecule GSi:
GSi
IUPAC name:
• 8-oxo-3,7-dihydro-[1,3,2,4,5] diazatrisilolo[4,5-d][1,3,2,4,5,6] diazatetrasilin-6-amine;
• PSA: 96.16;
• ALogP: 5.1999;
• Stereo Center Count: 0;
• Hydrogen Acceptor Count: 4;
• Hydrogen Donor Count: 3;
• Composition: H: 2.2% N: 30.3% O: 6.9% Si: 60.7%;
• Formula Weight: 231.50009;
• Exact Mass: 230.93404248;
• Molecular Formula: H5N5OSi5 [37].
TSi
Properties of molecule TSi:
IUPAC name:
• (2,4-dihydroxy-1,3,2,4,5,6-diazatetrasilinan-5-yl) silane;
• PSA: 64.52;
• ALogP: 8.6476;
• Stereo Center Count: 3;
• Hydrogen Acceptor Count: 4;
• Hydrogen Donor Count: 4;
• Composition: H: 5.7% N: 13.2% O: 15.1% Si: 66.1%;
• Formula Weight: 212.53497;
• Exact Mass: 211.974510294;
• Molecular Formula: H12N2O2Si5 [37] (Figure 2-5).
Conclusion and challenges
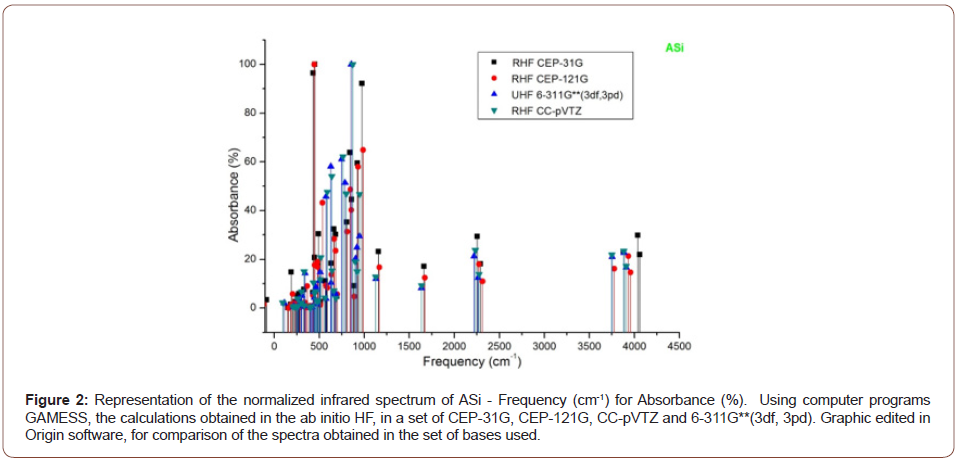
The absorbance peaks for Asi in the set of bases then used between 400 to 100 cm-1, 2250 cm-1 and 3750 to 4100 cm-1, (Figure 2).
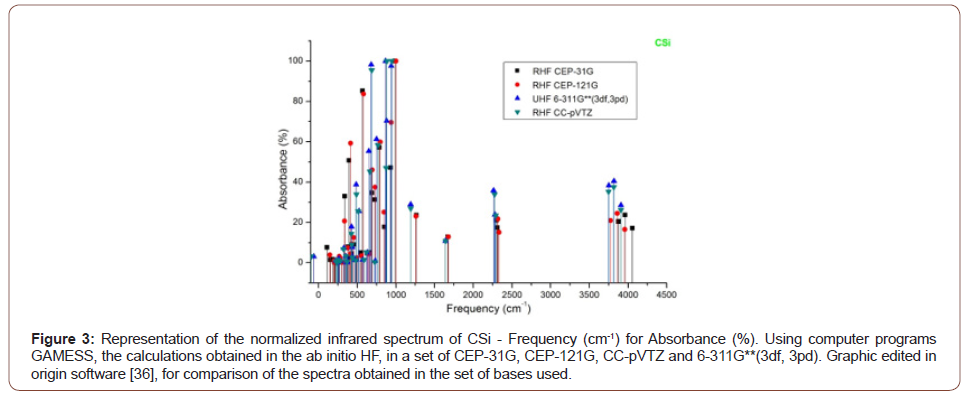
The absorbance peaks for Csi in the set of bases then used between 400 to 1000 cm-1, 2250 to 2300 cm-1 and 3750 to 4100 cm- 1, (Figure 3).
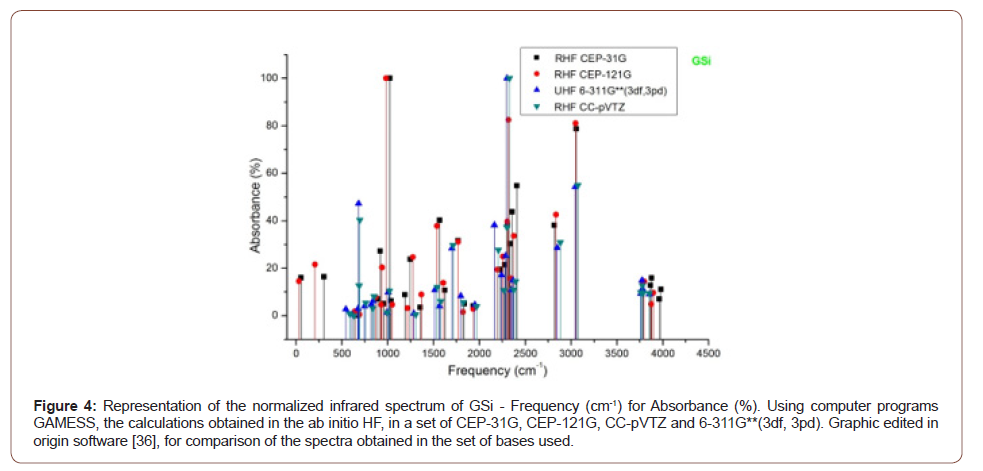
The absorbance peaks for Gsi in the set of bases used then between the range of 1000 cm-1, 2300 cm-1 and 3000 cm-1, (Figure 4).
The absorbance peaks for Tsi in the set of bases then used between 660 to 950 cm-1, 2250 to 2600 cm-1 and 3500 to 4000 cm-1, (Figure 5).
The fingerprint of the molecules ASi, CSi, GSi and TSI, are demonstrated in the Tables (1-4) and Figures (2-5).
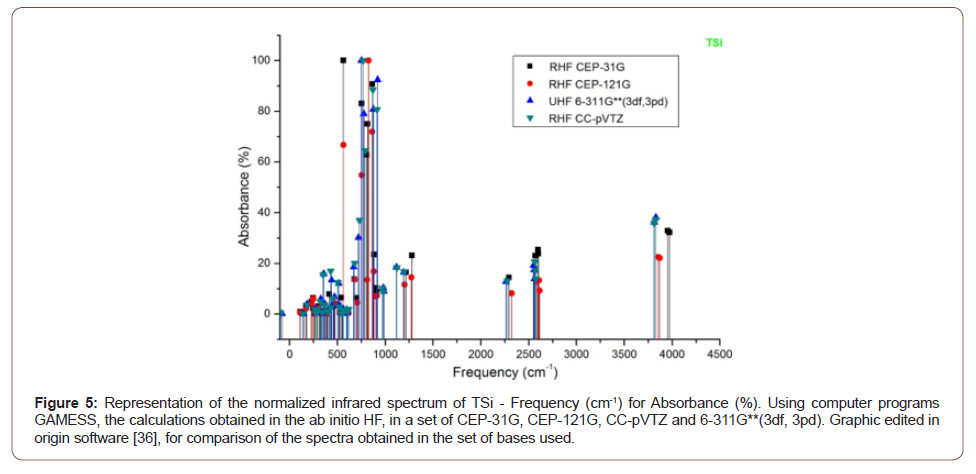
The infrared spectrum was calculated, indicating the characteristic of the nano-molecule genesis. Characterized its infrared spectrum, quantically calculated, accepted by quantum chemistry parameters, with ab initio methods, in the bases CC-pVTZ and 6-311G**(3df, 3pd). An experimental challenge to chemists.
Going beyond imagination, now the challenge is to build the basic structure of is the simulation for building a “new DNA helix”, based now on the ASi, CSi, GSi and TSi molecules. Limitations our study has so far been limited to computational simulation via quantum mechanics e molecular mechanics (QM/MM), an applied theory. Our results and calculations are compatible and with the theory of QM/MM, but their physical experimental verification depend on advanced techniques for their synthesis, obtaining laboratory for experimental biochemical.
To read more about this article....Open access Journal of Biomedical Engineering & Biotechnology
Please follow the URL to access more information about this article
To know more about our Journals...Iris Publishers





No comments:
Post a Comment