Abstract
Chemical as well as physical property that may occur under the influence of light is called Photochemistry. Photochemical transformations have been governed by two fundamental principles. These principals are First law: This law is also called as Grotthuss-Draper law; it states that for a photochemical reaction to take place it should be essential that light must be absorbed by a compound. Second law: The second law or Stark-Einstein law gives a photo equivalence law which is states that for each photon of light absorbed by a compound only one molecule of compound is activated for corresponding reaction. The efficiency of each photochemical process is calculated by Quantum Yield (Φ). Many photochemical reactions are complex, thus the quantum yield is specified for a particular event. It is defined as “ratios of the number of moles of a reactant disappearing, or the number of moles of a product obtained, per one mole of light absorbed by compound.” After that many of secondary reactions proceeded (shown in the gray box). Absorption of light (uv/vis) induces energy sufficient in molecule to break covalent bonds. Since, E = hc / λ, hence, longer wavelength have less energy and vice-versa. Consequently, ultraviolet light is most effect photochemical reactions. In this review we discuss about amongst all some of photochemical reactions collectively which are initiated by ultraviolet light specifically.
Keywords: Electromagnetic radiations; Singlet state; Triplet state; Excited state; Photochemical transformations
Introduction
In the early 1900’s Giacomo Ciamician from University of Bologna used sunlight for his research hence he is the father of photochemistry. Before that era, many sources used for a photochemical reaction these are bright incandescent lamps (chiefly infrared and visible light), low, medium and high pressure mercury lamps (185 - 255 nm, 255 -1000 nm & 220 -1400 nm respectively), high intensity flash sources and lasers. In careful studies of specific chromophore, sources of monochromatic light may be desired. In this review we focus on electronic excited states which are formed when a photon is absorbed by a chromophoric functional group present in molecule. Ultraviolet radiation having wavelengths less than 200 nm is sufficient to excite a electron to a higher energy orbital. A pictorial diagram showing the various kinds of possible electronic excitation in organic molecules is shown below (Figure 1).
All the six transitions outlined are achieved by the energies available in the 200 to 800 nm spectrum. Energetically favored electron excitation will be from the highest occupied molecular orbital (HOMO) to the lowest unoccupied molecular orbital (LUMO), and the resulting state is called an excited state. Electronic transition in the molecule is occurring only after; sufficient light energy will be absorbed. On excitation electron promoted to a higher energy orbital. The spectrum is drawn as a graph of absorbance (A) versus wavelength. Absorbance usually ranges from 0 (no absorption) to 2 (99% absorption), and is precisely defined in context with spectrometer operation. Franck-Condon Principle state that this electronic transition so faster than nuclei can respond. Bonding in an excited state is usually lower than in the ground state. Thus, bond length is increased in the excited state. Finely, electron in excited state may return to the ground state by emitting a photon (light blue line). This radiative decay is called fluorescence if it takes place rapidly from the initial excited state. It is termed phosphorescence if it occurs slowly by way of other excited states.
Excited states are of two types, singlet and triplet. This difference is just because of electron spin angular momentum. Most ground states are singlet, hence excited states initially formed by absorption of light is singlet. With loss of heat energy (relaxation), Internal conversion of excited states to lower energy states takes place. Also, an excited state may return back to the ground state via emitting a photon. The conversion of a singlet state to triplet state, or vice versa, is termed intersystem crossing. This process is slower than internal conversion. Radiative decay from a triplet state is called phosphorescence and is generally quite slow.
Types of electromagnetic radiation
Electromagnetic wave is only wave which is able to travel in empty space. Energy which is related with electromagnetic wave is called as electromagnetic energy and this is energy in the form of waves. Einstein and Max Plank said in his theory that Electromagnetic radiation exists in form of small packet of energy which is called photons. This energy behaves as waves and energy packets. Thus we can say that Electromagnetic energy is type of energy which originated from electromagnetic waves. This can also be defined as energy that transmits information (in the form of waves) from one place (material) to another or wave which is produced when charged particles that are placed in magnetic and electric field which are right angle to each other undergoes acceleration. The oscillation of the particles in the wave emits energy called electromagnetic wave energy. This information can be in the form of light, heat, or in any other form. Let us understand step by step what electromagnetic energy is. This energy radiation have same speed as speed of light and it contains radio waves, TV waves, radar waves, heat, light, X-rays, visible waves, etc. The Sun, the earth and the ionosphere are main sources of electromagnetic energy in nature (Table 1).
Table 1:Approximate Boundaries of Electromagnetic Radiations.

Some facts about electromagnetic energy
1. According to formulae E = hc / λ the higher the energy of the particles of electromagnetic wave, shorter is their wavelength.
2. It can travel through any material as well as through vacuum.
3. Their speed in vacuum is same as that of light, i.e. approximately 1, 86,000 miles per second or 3, 00,000 kilometers per second.
4. Most important thing is when it enter into matter, they get slow down i.e. their energy decreases, hence wavelength increases.
5. When this hits an object, it generates heat at the surface, this heat in turn causes the particles of that object to vibrate while in reverse when object is heated, particles get accelerated which causes change in their electric and magnetic fields, which leads to generate electromagnetic wave. Heat and vibration generated via this mode depends on the wavelength and energy of the electromagnetic wave (Figure 2).
Photochemical Reaction
Chemical reactions initiated by light are called as photochemical reactions. Energy in form of photon is absorbed or emitted by matter.
After absorption of light an electronic excitation from ground state to excited state takes place. This leads to promote an electron. The types of excitation are like n→π∗ or π→π∗ etc. Most of photochemical reactions take place during excitation from S1 and T1 excited states.
If a molecule absorbs energy, it can undergo photochemical reactions or there is possibility of they loss of energy via two methods: radiative processes which involve emission of a photon. Example of radiative phenomenon is phosphorescence which occur when electron relaxation to a lower state with different multiplicity, such as T1→S0 (spin forbidden). Other example is fluorescence in which relaxation occur to lower state of same multiplicity, such as S1→S0 (spin allowed). The non-radiative processes lead to no emission of photon. In this case the internal conversion occure which not involves spin change, such as S1→S0. The intersystem crossing-involves change in spin multiplicity. Excitation by energy transfer is Sensitization (deactivation is Quenching). There are lots of photochemical reactions. Some of these are discuss in this review.


1. Geometrical Isomerism: photochemical cis/trans (E/Z) isomerism in mostly leads to thermodynamically less stable cis-isomer -cis-isomer have less conjugation b/c of non-bonded interactions hence it typically absorbs at a lower λ [1] (Image 1).

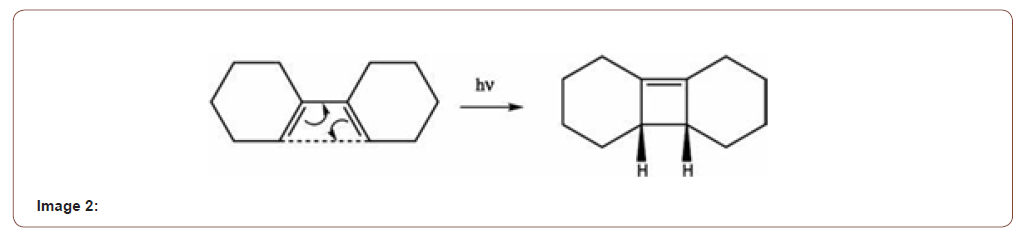
2. Electrocyclizations: Photochemical electrocyclization occur via LUMO 4n - disrotatory 4n+2- conrotatory. In this case a new σ-bond form between the termini of the conjugated π-system. Both bond breaking and bond formation process take place at the same time [2] (Image 2).
3. DeMayoReaction: In this reaction [2 + 2] cycloaddition takes place involving double bond of an enol and another olefin and the retro-aldol reaction [3] (Image 3).
4. Paterno-Büchi Reaction: Paterno and Chieffi observed the first example of a [2 + 2] cycloaddition between a carbonyl and an olefin to make an oxetane [4] (Image 4).
5. Arene-Olefin Cycloadditions: A photocatalyzed cycloadditions reaction between arenes and an olefin takes place all three positions available in arenes. On this basis these are summarized as ortho cycloadditions-[2 + 2], para cycloadditions-[4 + 2] and meta cycloadditions-[3 + 2] [5] (Image 5).



6. Photo induced Electron Transfer (WitkopCyclization): polar solvents facilitate the generation of radical ions and subsequent chemical reactions [6] (Image 6 & 7).
7. Photolysis of 3-hydroxy-3-methyl-2-butanone: photolysis of 3-hydroxy-3-methyl-2-butanone in presence of UV radiation leads to five major reaction products acetone, acetic acid, formaldehyde, CO and methanol [7] (Image 8).
8. Di-pi methane rearrangement: reaction involves the photolysis of molecule having two pi-bonds bonded to single sp3 hybridized C atom resulted into synthesis of cyclopropane [8] (Image 9).
9. Paterno buchi reaction: when carbonyl compound react with alkene in presence of light resulted in trimethylene oxide [9] (Image 10).
10. Photoinduced isomerization of azobenzene: the simplest photoinduced isomerisation is seen in azobenzene [10] (Image 11).
11. Norrish type II reaction: when photo-excited ketone abstract their H-radical from γ-position leads to corresponding biradicals, which is further resulted into cyclobutane is called Norrish type II reaction [11] (Image 12).







12. Novel photochemical rearrangement: 2-formyl phenylalkeno- derivatives in presence of UV light in benzene solution afforded thepolysubstituted isochromanones through Novel photochemical rearrangement [12] (Image 13).
13. Photo-Fries rearrangement: this photochemical reaction involves the homolytic cleavage of C–O, C–S and C–N, of esters, amides, thioesters etc [13] (Image 14).
14. Nitro-nitrite rearrangement: in this type of rearrangement nitro aromatic species undergoes photochemical initiated rearrangement [14] (Image 15).
15. Photochemical reaction of ethyl diazotrifluoropropanoate: photolysis of ethyl diazotrifluoropropanoate rearranges and resulted into efficient insertion reaction with the O–H bond [15] (Image 16).
16. Photochemical rearrangements of isoxazoles: this photochemical reaction afford acyl azirines, which resulted into the corresponding oxazoles [16] (Image 17).
17. 4,4-Diphenylcyclohexenone rearrangement: in such type of photo catalytic rearrangement one double bond and one of the phenyl groups, C-4, migrated to C-3 [17] (Image 18).
18. Photochemical rearrangements of Natural Product: the best result of such rearrangement is found in santonin which in presence of light converted into lumisantonin [18].
19. Photochemical Curtius rearrangement: in curtius rearrangement migration takes place along with full retention of configuration at R-group [19] (Image 19).
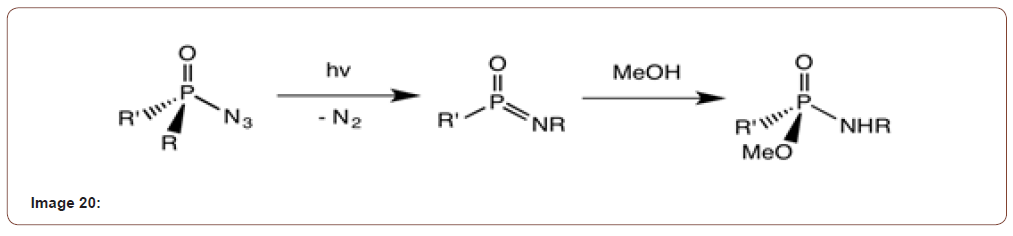
20. Photochemical Harger rearrangement: in Harger rearrangement the phosphinic azide forms a metaphosphonimidate in presence of light followed by methanol. This is actually R-groups which migrate [20] (Image 20).







Conclusion
Photochemistry itself is a mature science in this review, we have discussed about the unique features of photochemical reactions. A variety of photochemical reactions is described above. Overview of photochemical reaction also discusses significance of such reactions and is mainly focused on implications for main product. Ubiquity of photochemical reactions implies the importance of understanding the underlying processes and mechanisms on a molecular level. Now there is more need for new and exciting theory in this explored field. This is expected to be discovered in the future. It seems a promising field for future research.
To read more about this article....Open access Journal of Chemistry and Biochemistry
Please follow the URL to access more information about this article
To know more about our Journals...Iris Publishers





No comments:
Post a Comment