Authored by J William Louda*,
Introduction
Severe Microcystis aeruginosa blooms in Lake Okeechobee Florida occurred in 2016 [1] Oehrle, et al. (2017) Rosen, et al. (2017) and 2018 [2, 3]. Similar harmful algal blooms (HABs) occur worldwide and rapid assessment of phytoplankton communities is needed to trace of the onset, persistence and demise of such events. Additionally, changes in periphyton and epiphyte communities also require temporal and spatial monitoring.
Millie, et al. [4] stated “High-performance liquid chromatography (HPLC) has proven effective in rapidly separating and distinguishing chlorophylls, chlorophyll-degradation products, and carotenoids within monotypic and mixed algal samples. When coupled with absorbance and/or fluorescence spectroscopy, HPLC can accurately characterize phylogenetic groups and changes in community composition and yield information concerning microalgal physiological status, production, trophic interaction, and paleolimnology/ paleoceanography.”
While only in a very few cases can pigment analyses identify sources to the genus or species level (e.g. gyroxanthin diester from Karenia brevis), the identification to the Division level, or in certain cases to Class [5, 6], can be extremely helpful in monitoring ecosystem changes. The present overview is meant as an introduction to the methods and application of pigment-based chemotaxonomy [7].
Materials and Methods
Phytoplankton samples are routinely collected by inserting an inverted 1L amber bottle to 0.5m and rotating it to fill. The bottle is then sealed and placed on ice for transport to the lab. The use of other devices, such a Niskin or beta-water sampler, can be employed to collect samples at other depths for water column profiling.
Seagrass is harvested by hand while free (z<2m) or SCUBA diving. Seagrass blades are cut near their base with scissors and placed into pre-labelled (site, depth, date) large screw-top test tubes in order to capture any epiphytes that may slough off during handling and then are placed om ice for transport to the shore-based laboratory. Blades are measured for width (w) and length (l) to determine area (A cm2={2xw}xl). The blades are then gently scraped individually into an aluminum ‘pie plate’ using a polyethylene tissue lifter. The seagrass blades and the tissue lifter were then rinsed with water containing 3.5% salt (NaCl) by weight. The epiphytes are then collected by filtration as given below. Fake seagrass (epiphytometers) made of mylar strips fastened to weighted PVC piping and having a small piece of closed cell Styrofoam for blade tip flotation to mimic seagrass swaying in currents can be used as well. This allows an epiphyte mass per unit area estimation using total chlorophyll-a as a biomass proxy. Pigment-based chemotaxonomy then yields the community structure assessment [8].
Periphyton samples are very difficult obtain without also gathering/ extracting the host plants. Scrapping and/or sonication may be used. However, a better assessment of the periphyton communities in freshwater marshes can be obtained by using ‘periphytometers’. Basically, these are microscope slides which have been frosted by sanding with 200 or so grit sandpaper. These are then collected, scrapped akin to tat described for epiphytes above, collected, filtered and extracted [7].
Samples, whether environmental or cultured, are filtered through 47mm Whatman GF/F (0.7mm) filters. The filter is folded twice, blotted between paper towels, wrapped in aluminum foil, and frozen at -80oC until extracted within 24-28 hours. Samples are then extracted in a 10mL glass/Teflon homogenizing grinder while holding the tube in ice to minimize frictional heating during grinding. Extraction was with 5.0 mL of a mixture of Methanol, Acetone, Dimethylformamide, water [8].
One (1.00) mL of a crude extract is then mixed with 0.125 mL (125 L) of an ion-pairing solution [10] consisting of 15.0 g of tetrabutyl-ammonium acetate and 77.0 g of ammonium acetate per liter water total volume water. Prepared extracts are then injected using a Rheodyne 7125 injector and RP-HPLC was performed using a 3.9 x 300 mm Waters NovaPak 4-micron C18 column, developed with a ternary gradient [11] at 1.00mL/min. Solvents are delivered with a Thermo-Separations Products Model 4100 quaternary pump, and chromatograms plus absorption spectra are recorded using a Waters 996 PDA with Empower-2 software.
Numerous known chlorophyll and carotenoid pigments (e.g. Sigma-Aldrich{USA}, C14-DHI {Denmark}) and known phytoplankton species (e.g. UTEX, Bigelow Lab, Sigma-Aldrich) were purchased for QA/QC of chromatographic/spectrophotometric analyses. Copper- mesoporphyrin-IX-DME is used as an internal retention time and absorbance response standard as it does not coelute with any of the pigments of interest.
Results
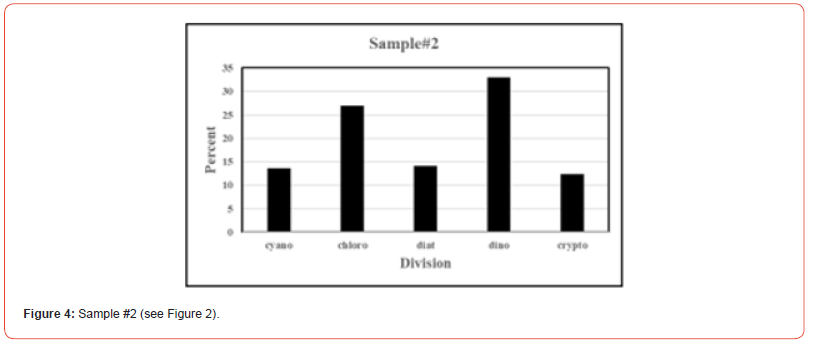
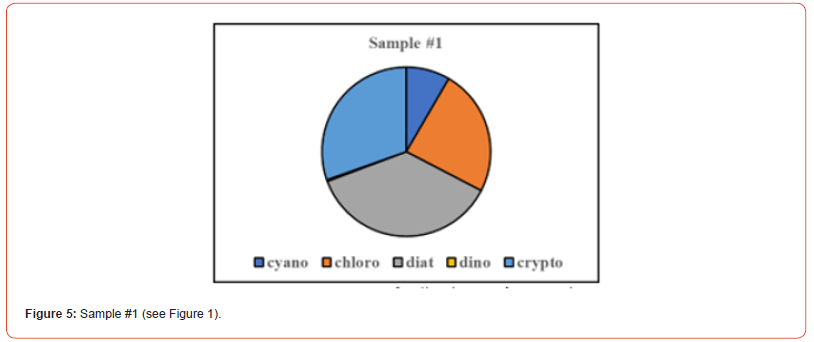
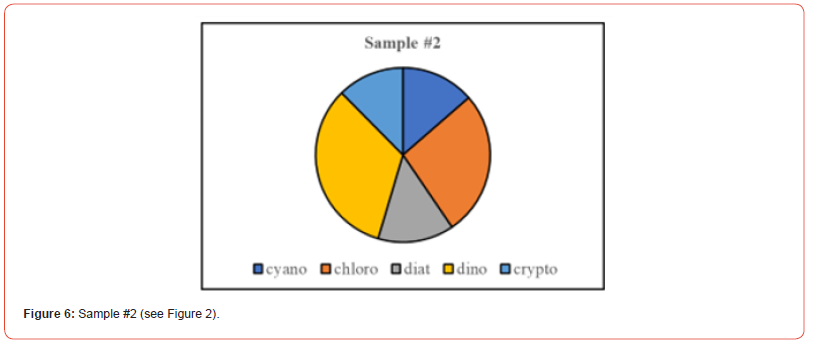
Two samples (Figures 1 & 2) of phytoplankton (microalgal) pigment-based chemotaxonomic assessments are given here. Both are from samples collected in an estuarine environment on the east coast of southern Florida.
Following the reversed phase high performance liquid chromatography (RP-HPLC) separation of the chlorophyll and carotenoid pigments, the analytical software, Waters Empower-2 in the present case, integrates all peaks at user selected wavelengths. Wavelengths used include 440nm for chlorophylls and most carotenoids, 410nm for pheopigments (Mg-free chlorophyll derivatives), 460nm for echinenone if coeluted with chlorophyll-a-epimer, and 664nm for chlorophyll-a-epimer when echinenone is present. It is noted that absorption maxima of many pigments do not fall exactly at 440nm and absorption corrections need to be made for proper quantification with know extinction coefficients. For example, the maximum absorption of the chlorophyll-a Soret band is at about 432nm so a correction of about 1.4x is required for data obtained at 440nm. Alternately CHLa can be measured at 432 or 662nm directly. Similar adjustments and QA/QC trial runs with known pure standard pigments should be made routinely.
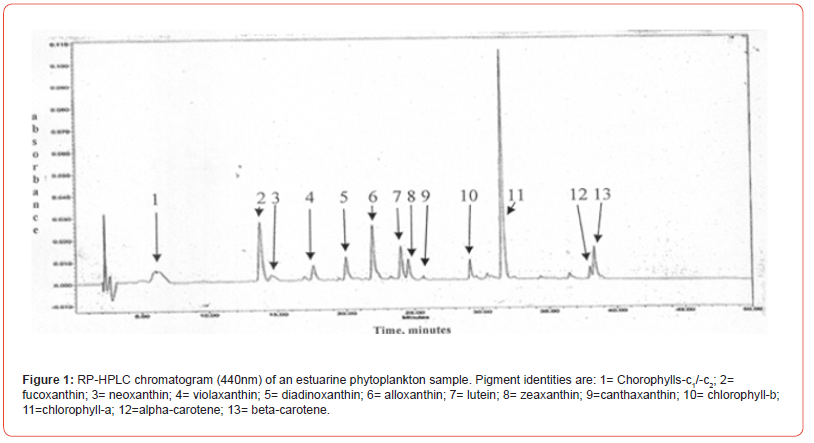
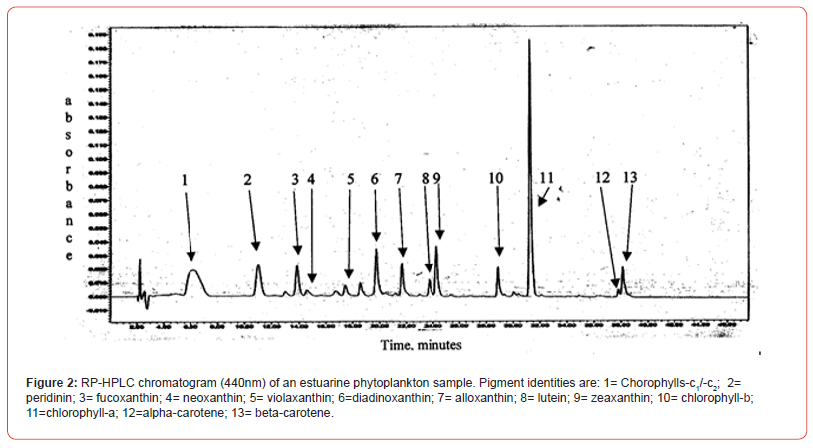
Pigment-based chemotaxonomic taxon (e.g. Division) estimation: Following integration of the peaks, the peak areas (as AU min) are entered into an Excel program for the calculation of pigment yield on both molar and weight bases. Then the amounts of specific biomarker pigments are used to calculate the amount of taxon-specific chlorophyll-a (CHLa). The pigments used for the five divisions considered herein are: zeaxanthin (Zea) for cyanobacteria, chlorophyll- b (CHLb) for chlorophytes, fucoxanthin (Fuco) for diatoms (Chrysophytes), peridinin (Peri) for dinoflagellates, and alloxanthin (Allo) for cryptophytes. It is noted here that the amount of zeaxanthin can be adjusted for minor contributions from other taxa such as chlorophytes. Other estimations may include separating coccoidal from filamentous cyanobacteria by using echinenone for the filamentous types, though many coccoidal types (e.g. Microcystis aeruginosa) also contain echinenone.
The mathematical treatment of pigment data can be performed using the CHEMTAX algorithm [5], the Bayesian Community Estimator [12], or Simultaneous Linear Equations [7, 9, 13]. Expanded details of each method can be found in Louda, et al. [7] and all the references cited therein.
An example of the SLE estimator based here on a molar rather than weight basis is:
CHLa = (1.1xZEA) + (2.4 x CHLb) + (1.2 x FUCO) + (1.5 x PERI) + (3.8 x ALLO)
Following such an estimation, a check on the efficiency of taxon- specific CHLa calculation can be made by comparing the estimated taxon CHLa with the total CHLa found in the RP-HPLC run. The output of the SLE estimation is given as percent (%) for each taxon, Divisions in the present case.
Table 1:
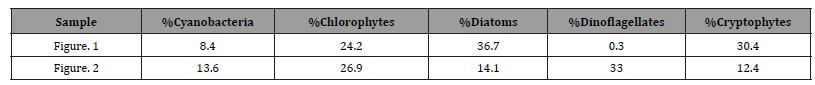
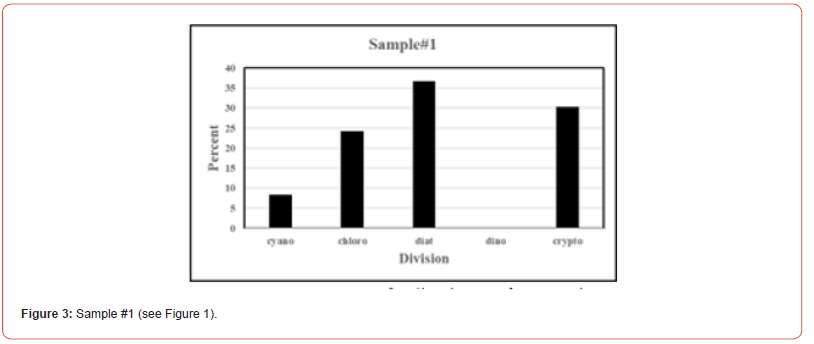
Once the percent taxa (Divisions) are calculated, various visual displays can obviously be made. This includes intera alia charts (Figures 3 & 4) and pie charts (Figures 5 & 6).
Discussion
Pigment-based chemotaxonomy for the rapid assessment of microalgal communities was introduced and reveiwed. The HPLC determination of pigments for the assessment of microalgal, notably phytoplankton, community structure see Chakraborty and Lohrenz, 2015 [7, 9, 14-16], Mackey, et al. (1986) [4,6,10,17-20], Detailed information on the pros and cons of microscopy versus HPLC pigment analyses for community assessment can be found in Havskum, et al. [21]. Certain caveats affecting this methodology need to be considered. The effect of varying light conditions which alter pigment ratios should be considered when assessing a microalgal community [22-24]. Pigment breakdown occurring during cellular senescence-death and/or heterotrophic processing (e.g. zooplankton feeding/fecal pellets) can alter observed ratios. This applies especially to chlorophyll-a itself [11, 25, 26] and therefore integration of the chromatogram at 410nm to calculate the amount of ‘pheopigments’ should be performed. Total CHLa should include chlorophyll-a, chlorophyll-a-epimer, chlorophyll-a-allomers, chlorophyllide- a and pyrochlorophyllide-a. The chlorophyllides are included here since the extract of viable diatoms can active their efficient chlorophyllase enzymes which cleave the phytol side chain.
Conclusion
Pigment-based chemotaxonomy has been shown to be an efficient methodology for the rapid assessment of phytoplankton (see Discussion above), epiphytes [8] and periphyton [7]. This method is especially adaptable to spatial/temporal monitoring and adaptive management scenarios [27].
To read more about this article...Open access Journal of Ecology & Environment Sciences
To know more about our Journals...Iris Publishers
To know about Open Access Publishers





No comments:
Post a Comment