Authored by Everton GA Melo*,
Introduction
In the past 12 years, multiquinase inhibitors, sorafenib and lenvatinib, represented the main first-line systemic treatments of hepato- carcinoma not amenable to local or metastatic treatment. The results of the phase III study, IMbrave 150, which compared atezolizumab plus bevacizumab versus sorafenib, represented an important advance in the first-line treatment. We report a clinical case of a 78-year-old patient with metastatic hepatocarcinoma, who underwent first-line treatment with the combination of atezolizumab and bevacizumab. After 3 treatment cycles, there was a complete response in the lung and a partial response in the liver, opening the discussion about the possibility of considering at some point the local treatment of the liver in the face of tumor downstaging.
Keywords: Hepatocarcinoma; Immunotherapy; Downstaging
Introduction
The incidence of hepatocarcinoma has been increasing con siderably in the population [1]. However, most cases (71%) diagnosed in the United States, are potentially preventable through obesity control, decreased use of alcoholic beverages, smoking cessation, prevention and treatment of hepatitis B and C [2]. In Brazil, hepatocarcinoma represents the 6th cause of death from cancer in men and 8th in women [3]. In the past 12 years, multiquinase inhibitors, sorafenib and lenvatinib, represented the main first-line systemic treatments of hepatocarcinoma not amenable to local or metastatic treatment. However, they have a modest survival gain and side effects that impact on patient’s quality of life [4, 5]. The combination of PD-L1 inhibitors and anti-VEGF therapies act in synergism, particularly in environments where high levels of VEGF are known to play an important role in tumor growth [6]. The results of the phase III study, IMbrave 150, which compared atezolizumab plus bevacizumab versus sorafenib, represented an important advance in the first-line treatment of patients with hepatocarcinoma not amenable to local or metastatic treatment [7]. This case report aims to present this new standard of treatment through the combination of therapies with PD-L1 inhibitors and anti-VEGF. Faced with the possibility of high response rates in 1st line treatment, we will have a new window of opportunity for the rediscussion of local treatments, such as radiotherapy and chemoembolization (TACE), after tumor downstaging.
Case Presentation
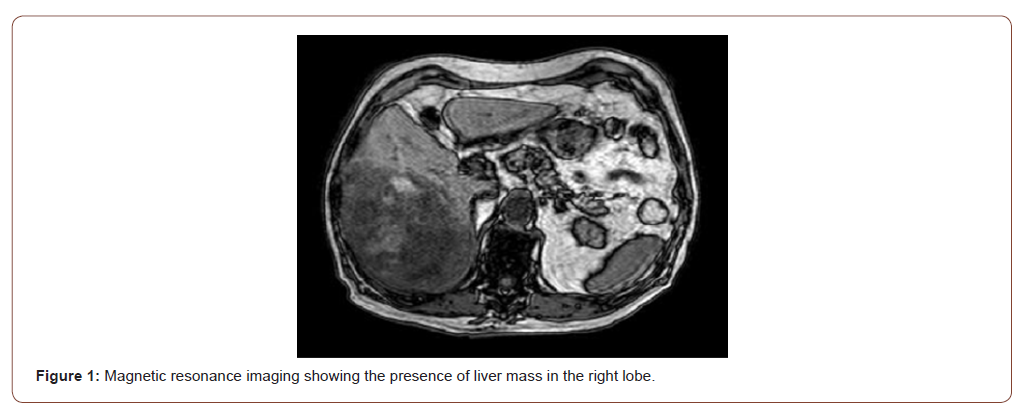
78-year-old male patient, with a past history of high daily alcohol intake (more than 3 doses of distillates), type 2 diabetes mellitus and hypothyroidism controlled with oral medications. About 4 months ago he started with significant weight loss (more than 10%) associated with pain in the right hypochondrium. In July 2020, he underwent an abdominal magnetic resonance exam that found the presence of a liver mass in the right lobe (Figure 1). In August 2020, he was taken to the emergency department due to acute abdominal pain associated with vomiting. After initial evaluation with imaging exams, the presence of an acute hemorrhagic abdomen secondary to a rupture of a liver tumor of about 20 cm was found. Thus, he was referred for emergency surgery and underwent a right hepatectomy. At the time, the anatomopathological condition was compatible with ruptured hepatocarcinoma.
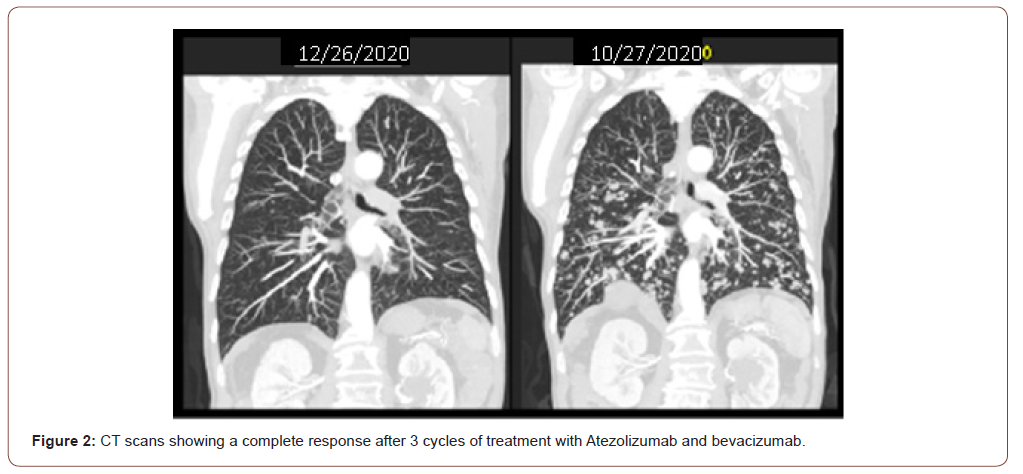
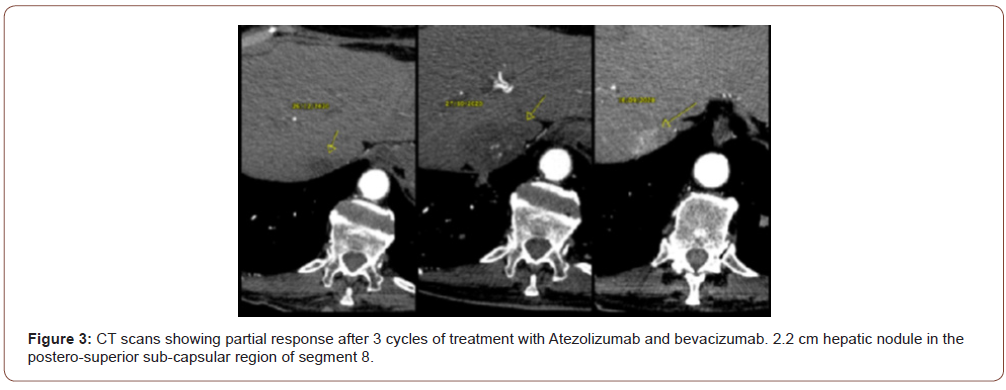
In the same hospitalization an Alpha Fetoprotein of 50,000 ng / ml was evidenced and in the staging exams multiple pulmonary nodules were found in the chest tomography and multiple liver nod- ules in the total abdomen tomography. Serologies for hepatitis B and C viruses were negative, upper digestive endoscopy showed the presence of thin esophageal varices not amenable to treatment with ligation and the CHILD score was A6. The patient was discharged with good performance (ECOG 1) and was classified with a BCLC score (Barcelona Clinic Liver Cancer Group) C. In October 2020, new CT scans were performed (10/27/2020) with the same findings and treatment with Atezolizumab 1200mg associated with Bevacizumab 15 mg / kg intravenously started every 3 weeks. After 3 cycles, he evolved with a significant clinical improvement, with no side effects related to the treatment and with new CT scans on 12/26/2020 showing a complete response of the lung lesions (Figure 2) and partial response of the liver disease (Figure 3), according to RECIST 1.1 image criteria. There was a 2.2 cm hepatic nodule in the postero-superior sub- capsular region of segment 8. The patient continues with the ap- plications every 3 weeks with a schedule of images to be taken every 3 months.
Discussion
The IMbrave150 phase III study demonstrated that treatment with atezolizumab plus bevacizumab achieved better response rates 27.3% (95% CI, 22.5 to 32.5) versus 11.9% (95% CI, 7.4 to 18.0), increase in median overall 12-month survival of 67.2% (95% CI, 61.3 to 73.1) versus 54.6% (95% CI, 45.2 to 64.0) and increase in median of progression-free survival of 6.8 months (95% CI, 5.7 to 8.3) versus 4.3 months (95% CI, 4.0 to 5.6) when compared to sorafenib in patients with advanced hepatocarcinoma undergoing first-line treatment. Serious toxic effects were observed in 38% of patients who received the combination therapy, however, no new or unexpected toxic effects related to the combination. Finally, the combination therapy also resulted in a longer time for the deterioration of the quality of life [7].
A paradigm shift has been taking place in the treatment of metastatic hepatocarcinoma, because, until then these patients were candidates for sequential systemic therapy and rarely underwent local treatments, such as radiotherapy or TACE. The hypothesis that bevacizumab increases the activation of CD8 + T cells by reversing the immunosuppressive effects of VEGF and in association with Atezolizumab increases the cytotoxic effects against cancer cells [8,9], causes us to come across higher rates of objective response. Thus, new treatment perspectives should be discussed based on tumor downstaging. In the era of effective immunotherapy-based treatments for patients with metastatic hepatocarcinoma, it will be important to select which patients will remain with exclusive systemic treatment and which may be reconsidered for local treatment in the presence of a complete distance disease response and maintenance of residual liver disease.
There are several clinical studies underway based on immunotherapy strategies combined or not with local treatments [10]. From these results we will have new data that will expand the discussion on the best treatment strategy to be adopted. The fact is that these new efficient options in the treatment of hepatocarcinoma bring great hopes and benefits to patients who in the past 12 years have been waiting for more promising treatment strategies.
Conclusion
The results of the IMbrave 150 study establish the combination of atezolizumab plus bevacizumab as the new treatment standard for hepatocarcinoma not amenable to local therapy or metastatic. Thus, we will start new discussions about the indication of radiotherapy or TACE for patients who have a complete response of metastatic lesions.
To read more about this article...Open access Journal of Gastroenterology & Hepatology
Please follow the URL to access more information about this article





No comments:
Post a Comment